Figure 6.
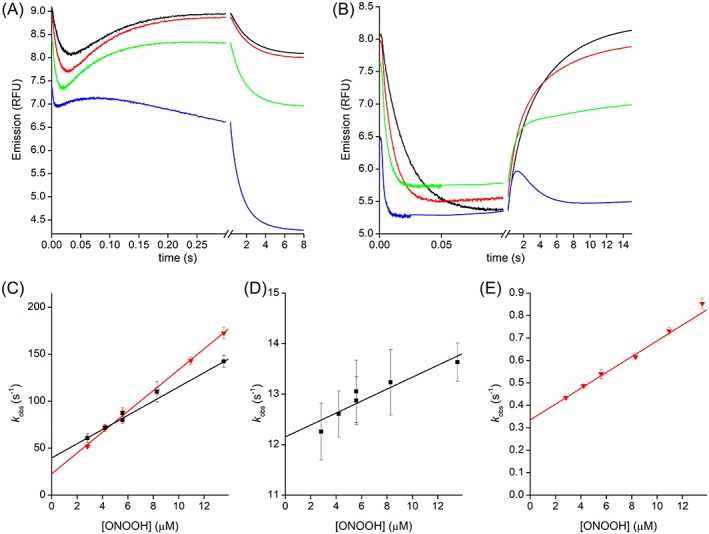
Kinetics of PRDX1 and PRDX2 oxidation by ONOOH. Time courses of the oxidation of PRDX1 (a) or PRDX2 (b) with excess ONOOH, from top to bottom 2.8, 8.3, 13.5 and 71 μM, and traces are averages of 15 runs. (c) Second‐order plot of the rate constant of the fast exponential descending part of the time courses for PRDX1 (black squares) or PRDX2 (gray triangles). Second‐order plot of the rate constant of the ascending exponential part of the time courses for PRDX1 (black squares, d) or PRDX2 (gray triangles, e). General conditions: buffer A, pH 7.3, 25 °C, λex = 280 nm, λem > 320 nm.
