Figure 1.
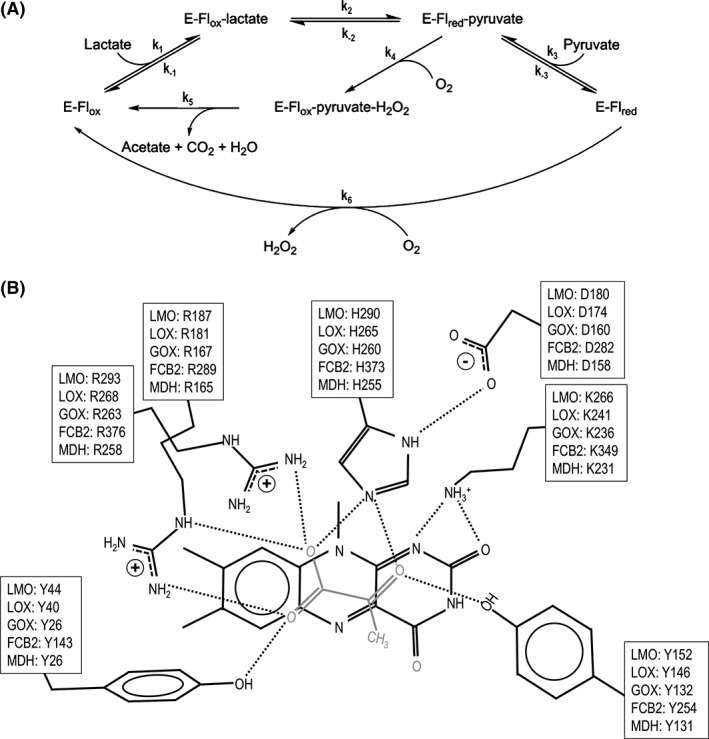
The LMO/LOX catalytic cycles and conserved active site of α‐hydroxy acid oxidases. (A) The inner loop represents the “coupled pathway” observed for LMO where pyruvate is an intermediate and acetate and CO2 are the final products. The outer loop represents the “uncoupled pathway” observed for LOX where pyruvate and H2O2 are produced by two uncoupled half‐reactions. E, Flox, and Flred represent the enzyme, oxidized flavin, and reduced flavin, respectively. Rate constants defined for each step are indicated. Adapted from Ref. 27. (B) Seven conserved residues in the active site of LMO, LOX, GOX, FCB2, and MDH are shown in front of the si‐face of the flavin along with pyruvate (grey) as it is seen bound to LOX in PDB entry 2E77. Typical hydrogen bonds (dotted lines) are also shown. The residue numbers are based on M. smegmatis LMO, and PDB entries 2E77 (LOX), 2RDU (GOX), 1KBI (FCB2), and 1P4C (MDH).
