Figure 7.
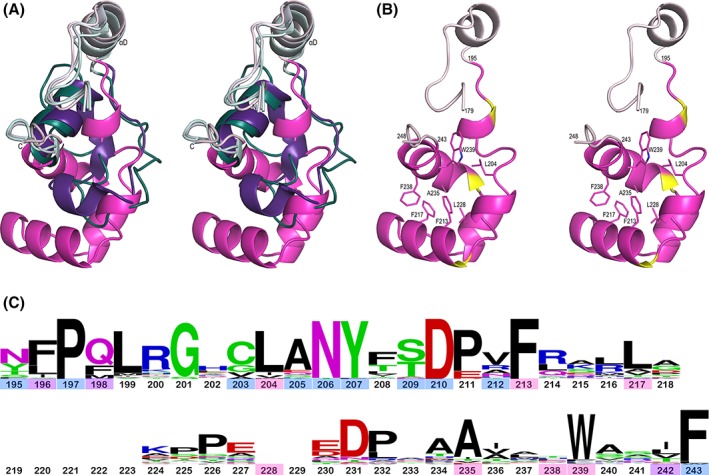
Comparison of Loop 4 in LMO, LOX, and GOX. (A) Stereoview of the chain between β4 and α4 of the LMO monomer (pink tones) overlaid with the LOX–pyruvate complex (purple tones; PDB entry 2E77) and the GOX–glyoxylate complex (teal tones; 2RDU). The N‐ and C‐termini are marked and segments between β4 and α4 are highlighted with the more spatially conserved parts at the beginning and end, including αD, in light colors and the varying Loop 4 portion in dark colors. The LOX and GOX Loop 4 segments follow similar paths to each other. (B) Stereoview of the LMO chain between β4 and α4 (orientation and ribbon coloring as in A, but with three helix‐capping Pro residues in yellow) with seven central core side chains shown as sticks and labeled. (C) WebLogo plot based on Loop 4 sequences from 48 representative LMOs using M. smegmatis LMO numbering. Residue numbers are colored based on the side chain role in packing: central core of Loop 4 (pink), interface between Loop 4 and the rest of LMO (blue), or both core and interface (purple). At 49 residues long, M. smegmatis LMO has the longest Loop 4 with the most common length being 42 residues; the 7 residues not present in most LMOs are blank spots in the WebLogo. The conservation of Gly201 which adopts a glycine‐preferred ϕ,ψ‐conformation and Pro197, Asp210/Pro211, and Asn231/Pro232 which form helix N‐capping motifs imply a strong conservation of these structural elements across all putative LMOs. The very conserved Asn206 and Tyr207 pack at the interface with the rest of LMO, with the Asn206 side chain hydrogen bonding to backbone atoms 135‐N and 129‐O, and Tyr207 packing between Phe163 and Loop 4 core residues.
