Figure 6.
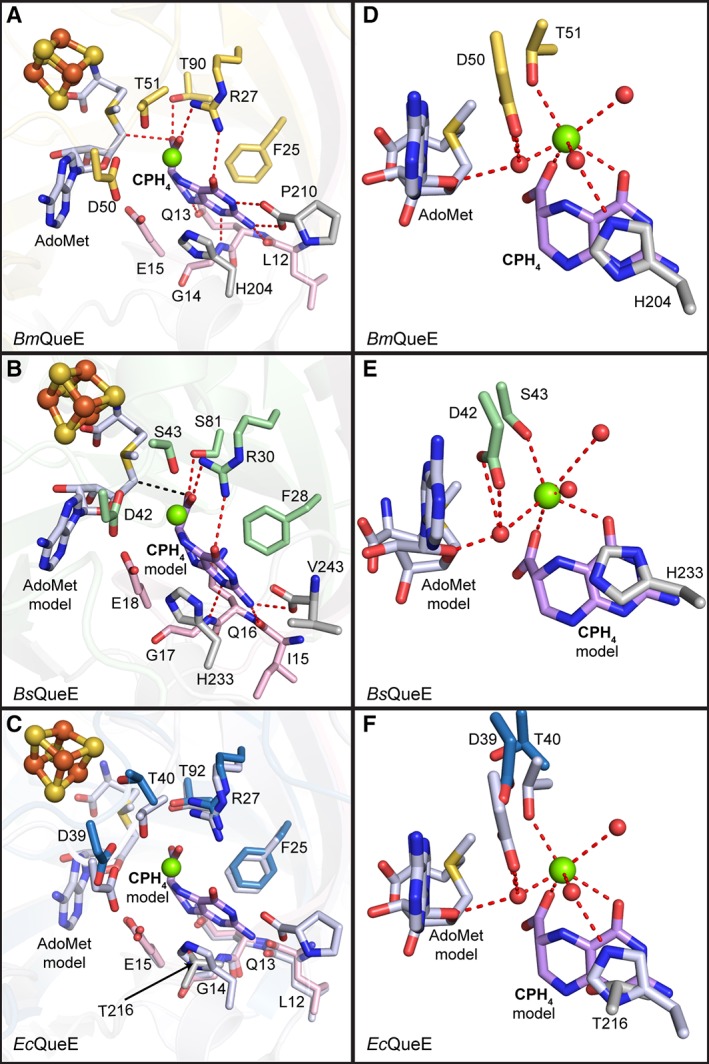
Substrate binding pocket. Residues (in sticks) that comprise the substrate‐binding pocket are shown for each of the QueE homologs. (A) CPH4 is bound to the active site by residues from the N‐terminal extension (pink), the AdoMet radical core fold (yellow) and the C‐terminal extension (grey) of BmQueE (PDB ID 4NJI). (B) In the modeled orientation, CPH4 appears to interact with the AdoMet radical domain (green) of BsQueE in addition to the N‐ and C‐terminal extensions, colored pink and grey, respectively. (C) CPH4 modeled into EcQueE. AdoMet radical domain in blue and N‐ and C‐terminal extensions in pink and grey, respectively, are shown overlaid with the active site of BmQueE (white). (D) CPH4 binding in BmQueE (PDB ID 4NJI) (yellow) creates a magnesium‐binding site. (E) CPH4 binding in BsQueE (PDB ID 5TH5) (green) is expected to create a magnesium‐binding site similar to that seen in BmQueE. (F) The putative magnesium‐binding site of EcQueE (blue) is shown overlaid with the CPH4 bound BmQueE (PDB ID 4NJI) (white). The substrate, CPH4, is shown in lilac, the catalytically essential magnesium is represented as a green sphere, the irons (orange) and the sulfurs (yellow) of the [4Fe–4S] AdoMet radical cluster are shown as spheres, AdoMet is shown in light blue and hydrogen bonds are shown as red dashes. Water molecules (red spheres) necessary for magnesium binding are shown.
