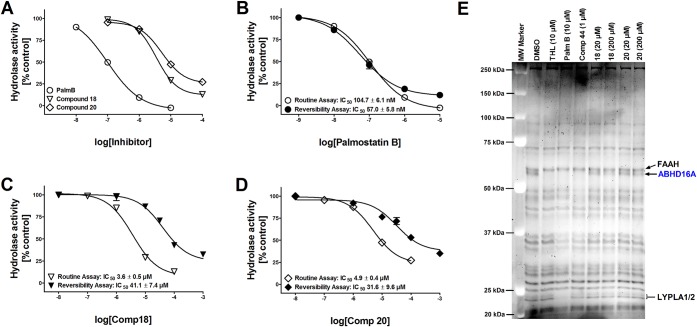
Figure 2.
Biological characterization of 18 and 20 in comparison with palmostatin B. (A) Dose–response curves for palmostatin B, 18 and 20 to inhibit 1-LG hydrolysis in lysates of hABHD16A-HEK cells. Lysates were pretreated for 30 min with DMSO (control) or with the indicated concentrations of the inhibitors before adding the substrate (25 μM final concentration). Glycerol liberated from 1-LG hydrolysis was determined as previously described.6 Note that in contrast to 10 μM palmostatin B, which comprehensively blocks 1-LG hydrolysis, ∼20% and ∼30% residual activity remains at 100 μM concentration of 18 and 20, respectively. Values are mean ± SD of duplicate wells from two independent experiments. (B–D) Reversible nature of hABHD16A inhibition by 18 and 20. Fast 40-fold dilution of inhibitor-treated hABHD16A-HEK293 lysate preparation in the reversibility assay results in notable drop of the diterpene inhibitor potency, as compared to potency values obtained using the routine assay. In contrast, the potency for the β-lactone palmostatin B remains similar during the time-course of this study. Data are mean ± SD from two independent experiments. (E) Competitive ABPP using rat cerebellar membrane proteome reveals ABHD16A as the sole serine hydrolase targeted by 18 and 20. THL (10 μM), palmostatin B (10 μM), and compound 44 (1 μM) were used as positive controls to identify ABHD16A and in line with our previous study,6 all three blocked TAMRA-FP labeling of a band migrating at ∼63 kDa, corresponding to ABHD16A. Compound 18 dose-dependently inhibits TAMRA-FP labeling of ABHD16A, whereas 20 was marginally effective only at the higher concentration. Note that in contrast to THL and palmostatin B, no additional targets are evident for 18 or 20 among the metabolic serine hydrolases. The gel is representative of two independent ABPP runs with similar outcome. FAAH, fatty acid amide hydrolase; LYPLA1/2, lysophospholipase A1/A2.