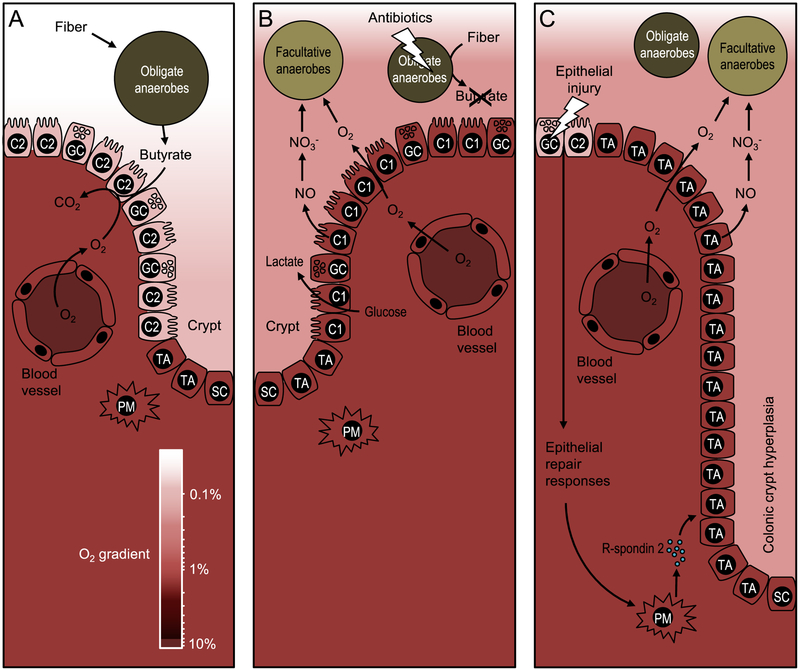
Figure 1: Epithelial metabolism shapes the colonic microbiota.
(A) During gut homeostasis, obligate anaerobic bacteria convert fiber into fermentation products, such as butyrate, to maintain differentiated colonocytes in a C2-skwed metabolic state. The metabolism of C2-colonocytes is characterized by high oxygen consumption, which maintains epithelial hypoxia (<1 % oxygen) to limit the amount of oxygen diffusing into the gut lumen. The color scale on the bottom indicates oxygen (O2) levels, which are between 3 % and 10 % in normoxic tissue (85). (B) Disruption of the gut microbiota by antibiotic treatment depletes microbe-derived fermentation products, causing a metabolic reorientation of terminally differentiated colonocytes towards a C1-skewed metabolism, which is characterized by high lactate release, low oxygen consumption and elevated synthesis of iNOS, an enzyme that generates nitric oxide (NO). Conversion of nitric oxide into nitrate (NO3−) in the gut lumen together with oxygen (O2) emanating from C1-colonocytes provide electron acceptors that drive an expansion of facultative anaerobic bacteria. (C) Epithelial injury activates epithelial repair responses, including a release of R-spondin-2 to stimulate cell division of undifferentiated transit-amplifying cells. Excessive cell division of undifferentiated transit-amplifying cells leads to colonic crypt hyperplasia and an increased epithelial oxygenation. Nitrate and oxygen emanating from the mucosal surface during colonic crypt hyperplasia drive an expansion of facultative anaerobic bacteria. PM, pericryptal myofibroblast; SC, stem cell, TA, undifferentiated transit-amplifying cell; C2, terminally differentiated C2-colonocyte; C1, terminally differentiated C1-colonocyte; GC, goblet cell.