Figure 2. Key elements of GPCR target validation.
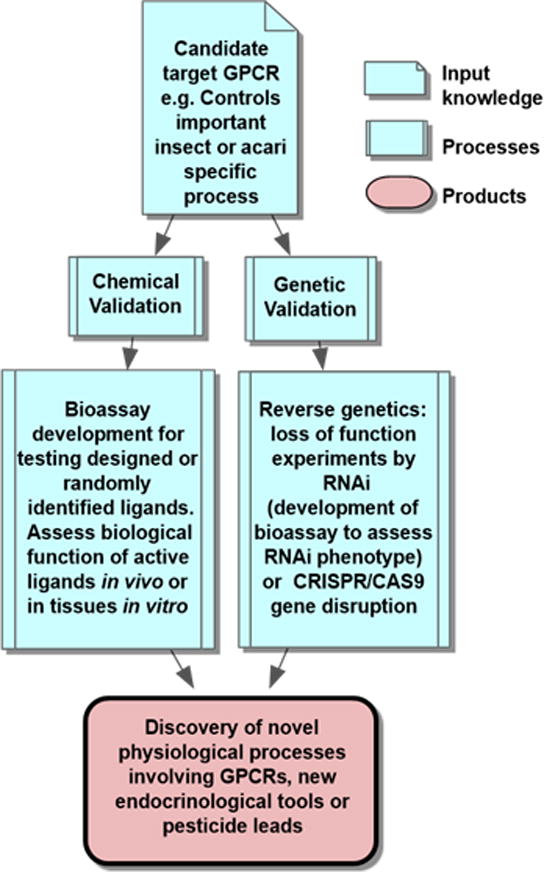
For chemical validation of a GPCR as a candidate target, the designed or identified ligand that is active on the recombinant receptor is applied in vivo and in vitro in tissues. Bioactivity must be determined, either as mortality or by another adverse biological effect derived from its action as antagonist or superagonist (i.e. paralysis). For genetic validation, “loss of function” experiments most typically, or “gain of function” experiments, must confirm the disruption of receptor function has a measurable effect. Through these processes, we validated stable potent peptidomimetics for, and discovered novel functions of the kinin receptor [73].
