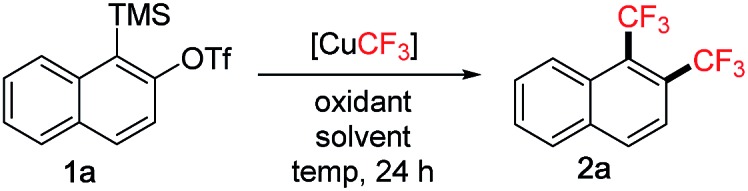
Table 1. Optimization studies for 1,2-bis(trifluoromethylation) of aryne precursor 1a a .
| ||||
| Entry | Oxidant | Solvent | Temp. (°C) | Yield b (%) |
| 1 c | Air | DMF | 50 | 30 |
| 2 | None | DMF | 50 | 0 |
| 3 | BQ | DMF | 50 | 4 |
| 4 | Cu(OAc)2 | DMF | 50 | 7 |
| 5 | AgOAc | DMF | 50 | 26 |
| 6 | PhI(OAc)2 | DMF | 50 | 26 |
| 7 | DDQ | DMF | 50 | 58 |
| 8 d | DDQ | DMF/DMSO | 50 | 77 |
| 9 d | DDQ | DMF/DMSO | rt | 78 |
| 10 e | DDQ | DMF/DMSO | rt | 62 |
| 11 f | DDQ | DMF/DMSO | rt | 77 |
aUnless specified otherwise, reactions were carried out using 1a (0.1 mmol), [CuCF3] (0.4 mmol in 1.0 mL DMF), oxidant (0.2 mmol) and DMF (1.0 mL), under argon.
bDetermined by 19F NMR analysis using benzotrifluoride as the internal standard.
cReaction was open to air.
dDMF : DMSO = 1.0 : 1.0 mL.
eDMF : DMSO = 1.0 : 0.5 mL.
fDMF : DMSO = 1.0 : 2.0 mL.
