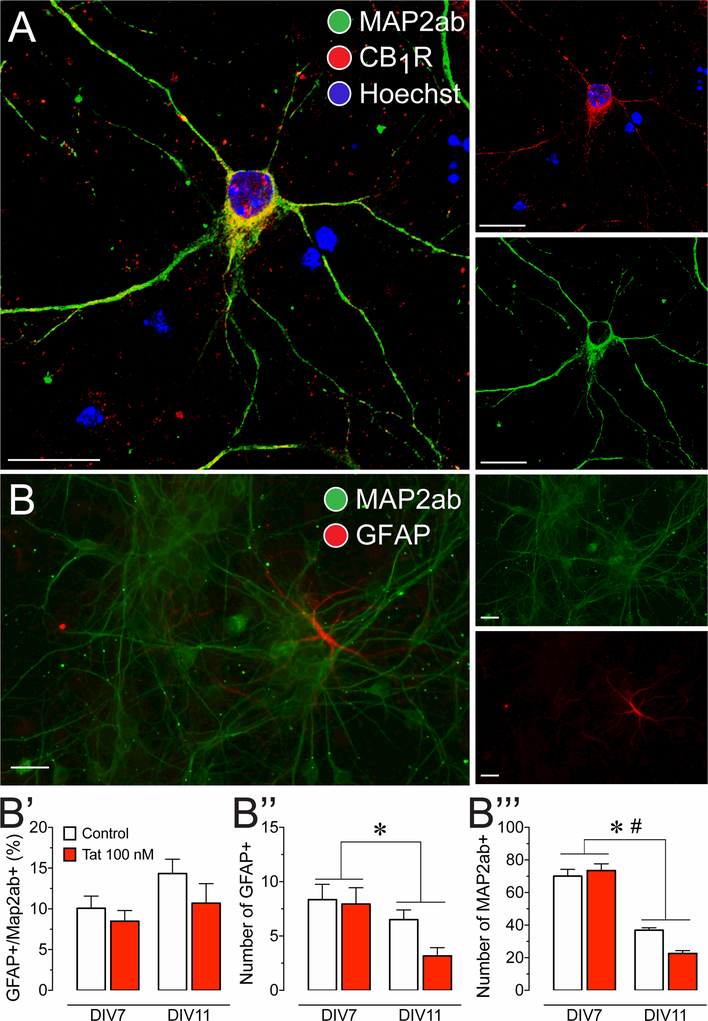
Figure 1. Presence of CB1R in primary dissociated PFC neuronal cultures at day in vitro 7–11.
(A) Immunohistochemistry was conducted on PFC neurons (DIV 7–11) to identify cellular distribution of CB1R. Cells were stained for Map2 (green) and endogenous CB1R (red), and imaged using the Zeiss LSM 700 confocal microscope. CB1R are localized in the soma and dendritic processes of PFC neurons, indicating that the cannabinoid machinery is present in our PFC primary cell culture model. (B) Immunohistochemistry was conducted on PFC neuronal cultures to calculate a ratio of glial cells to neurons. Cells were stained for Map2ab (green) and GFAP (red), and imaged using the Zeiss Axio Observer Z.1 microscope. (B’) GFAP-positive cells with astrocyte morphology were present in our PFC cultures and made up about 11% of the cultured cells. The percent astroglia composition was not significantly altered by Tat 100 nM incubation and/or time (DIV 7–11). Independent assessment of astroglial (B”) and neuronal (B’”) populations revealed a significant reduction in both populations over 4 days in vitro (DIV 7–11). Further, for Map2ab-positive neuronal population a significant time by Tat interaction was noted that further decreased neuronal cell count. Statistical significance was assessed by two-way ANOVAs (at least three independent experiments), *p < 0.05 main effect of time, #p < 0.05 time x Tat interaction. Scale bars = 20 μm.