Abstract
Introduction
Adolescents and young adults (AYAs) diagnosed with cancer between ages 15-39 years may harbour germline variants associated with cancer predisposition. Such variants represent putative therapeutic targets, as may somatic variants in the tumour. Germline and tumour molecular profiling is increasingly utilised to facilitate personalisation of cancer treatment in such individuals.
Aim
Considering AYAs with advanced solid tumours managed in a specialist drug development unit (DDU), the aims of this study were to investigate the use and impact of:
Germline genetic assessment
Tumour molecular profiling
Methods
AYAs treated in the DDU at the Royal Marsden Hospital between 2002 and 2016, were identified from departmental databases. Data regarding clinicopathological features, clinical assessments, germline and tumour genetic testing were retrieved by chart review.
Results
The study cohort included 219 AYAs. Common cancer types included sarcoma (41, 19%); cervical (27,12%); breast (25; 11%); ovarian (23,11%) and colorectal (21,10%) cancers. Germline testing was undertaken in 34 (16%) patients, 22 of whom carried a pathogenic variant. Using current testing criteria, an additional 32 (15%) would be eligible for germline testing based on their personal history of cancer alone. Tumour testing was undertaken in 46 (21%) individuals. Somatic mutations were commonly identified in TP53 (12,31%), PIK3CA (8,18%); KRAS (4, 9%) and MET (4,9%).
Discussion
A significant proportion of AYAs with advanced cancer have targetable somatic or germline mutations. Consideration of familial risk factors, and inclusion of germline testing where appropriate can complement tumour testing to optimize patient management, and inform management of at-risk relatives.
Introduction
The aim of phase I clinical trials is to determine the safety and tolerability of experimental agents in humans(1). Because of the unpredictable side effect profile and indeterminate efficacy of these agents, the majority of patients referred for consideration of such trials generally have advanced stage, heavily pre-treated tumours, or rare tumours with a paucity of available standard therapies. To fulfil eligibility criteria for such trials, patients must be of acceptable medical fitness (ECOG* 0 or 1) (2)), such that they have enough physiological reserve to cope with unexpected side effects. The patient population of phase I units is therefore enriched for younger patients, despite the relative rarity of cancer in individuals under the age of 40. Other factors are also considered in determining eligibility for phase I trials, including previous therapies and current medications and co-morbidities. Although the aim of the Phase I trial is to determine safety of experimental agents, patients are preferentially allocated to trials with best predicted likelihood of efficacy based on pre-clinical evidence, given that patient outcomes are known to be better when therapy is targeted (3–5). Molecular characterisation of tumours by sequencing or by immunohistochemistry may inform decision-making with respect to allocation to a trial of a targeted agent, immunotherapy or anti-angiogenic agent depending on the molecular defect identified(6, 7). As genomic technologies have improved, and become more affordable(8), the methods by which this molecular characterisation can be routinely undertaken have changed dramatically in our institution, moving from hotspot mutational analysis by PCR to sequencing by next generation technologies using multi-gene panels. Our panels have also expanded over time to reflect increases in the size of the portfolio of targeted agents under investigation in our unit. The panel currently in use in our unit includes 113 “DNA damage repair” genes, including tumour suppressor genes such as BRCA1, BRCA2 or mismatch repair genes.
Individuals diagnosed with cancer between the ages of 15 and 39 are referred to as “adolescents and young adults (AYAs)(9). Cancers occurring in this cohort may include paediatric cancers occurring at unexpectedly older ages, or cancers of adulthood occurring at unexpectedly young ages(10–12). Such phenomena are characteristic of inherited cancer predisposition syndromes. Certain cancer types (13)occurring in this age group are also strong predictors of an underlying germline defect. The approximate likelihood of identifying a pathogenic mutation in TP53 in an AYA with an adrenocortical cancer, for example, is 13%(14), which is lower than the probability of identifying a mutation in a child with the same cancer (50-80%)(15–17), but over twice that of older adults (5.8%) (18). The characteristic cancer occurring in individuals with CDH1 mutations is diffuse gastric cancer, and the mean age of diagnosis in affected individuals is 38-40 years(19, 20). The association between high grade serous ovarian cancer and triple negative breast cancer with BRCA1/BRCA2 mutations is well-established(21–23), and the likelihood of detecting a mutation in one of these genes in an affected individual is inversely proportional to the age at diagnosis. For this reason, in our institution, germline genetic testing is offered to individuals diagnosed with these and other certain types of cancer at ages younger than 40 years, irrespective of family history (Table 1). For other tumour types, tumour-based immunohistochemical analysis is recommended. In 2017, the NICE guidelines were updated to include a recommendation that all individuals diagnosed with colorectal cancer should have analysis of their tumour by IHC for mismatch repair proteins, or microsatellite stability testing, to guide further testing for Lynch syndrome(24). In our institution, routine IHC is also performed on all endometrial cancers for the same purpose.
Table 1. Indications for genetic testing in our unit.
| Cancer | Gene | Indication for testing |
|---|---|---|
| Breast | BRCA1/2 | All Patients <45y |
| TP53 | All Patients <30 if BRCA1/2 testing uninformative | |
| Colorectal | MMR IHC± germline analysis | All patients |
| APC/MUTYH | CRC + ≥3 adenomatous polyps | |
| MUTYH | CRC + 2 affected sibs | |
| Sarcoma | TP53 | Rhabdomyosarcoma <5y Other sarcomas, depending on family history |
| Adrenocortical cancer | TP53 | All patients |
| Thyroid | RET | All medullary thyroid cancers |
| PTEN | Depends on clinical features | |
| Kidney | Renal Panel | All patients <40y |
| Paraganglioma/Pheochromocytoma | PGL/Phaeochromocytoma panel | All patients |
| Ovarian | BRCA1/2 | All epithelial ovarian ca |
| Stomach | CDH1 | All patients <45y |
| Endometrial | MMR IHC± germline analysis | All patients |
| FH/PTEN | Depends on clinical features |
We conducted a study of AYA patients undergoing phase I clinical trials at the Drug Development Unit, Royal Marsden Hospital UK. One aim of this study was to investigate the proportion of individuals in this cohort with inherited cancer predisposition syndromes, and to identify how many additional individuals in this cohort would be eligible for germline investigations using current testing criteria. A second aim of this study was to examine the utility of germline and somatic testing in determining trial allocation in this patient cohort.
Methods
Germline Genetic Assessment
In recent years, germline genetic testing at our institution has been performed using the Illumina TruSight cancer predisposition gene panel. After library preparation, sequencing is performed on the Illumina HiSeq2500. Although all genes on the panel are sequenced, only data pertaining to the genes of interest according to the clinical request form are analysed. Sequencing data is analysed using a bespoke pipeline. Rare and/or pathogenic variants are confirmed by Sanger sequencing or Multiplex Ligation-dependent Probe Amplification (MLPA)(25). Testing in other units utilises similar next generation sequencing technology. Prior to the availability of next generation sequencing, sequencing of single genes was generally performed by Sanger Sequencing and MLPA. Currently, 90% of results from diagnostic BRCA1 and BRCA2 analysis are reported within three weeks, and 80% of results from diagnostic analysis of other genes within four weeks(26).
Somatic Genetic assessment
Testing of tumours for potentially targetable mutations has been undertaken in our unit since 2011, commencing initially with testing of tumours for recurrent hotspot mutations in known driver genes, followed by sequencing of limited numbers of genes, and moving more recently to next generation sequencing of tumours using multi-gene panels. Tumours from twenty-eight patients were analysed using a 48-gene TruSeq® Amplicon Cancer Panel, and tumours from eleven patients were analysed using a custom-designed 113-gene “DNA damage repair” panel. Tumours from seven other patients were tested using multi-gene panels, but reporting of results was limited uniformly to ATM, BRAF, EGFR, HRAS, KRAS, NRAS, PIK3CA, and TP53, and variably to other genes of interest depending on the cancer type. In addition, select patients underwent immunohistochemical assessment of PTEN and ATM. For the purposes of this study, only results from multigene panel testing were analysed. The selection of patient to whom somatic testing was offered was largely dependent on portfolio of trials available within the unit at the time of the patient referral. Tumour molecular characterisation was offered to those patients for whom eligibility for a trial depended on presence/absence of particular molecular aberrations.
Compared to germline variants, where the American College of Medical Genetics have defined strict guidelines for determination of pathogenicity, there is a paucity of guidance with respect to classifying somatic variants. Sequencing data were analysed using a bespoke pipeline, and variants identified with frequency greater than 5% were reported. Variants were classified as “high”, “medium” or “low” impact depending on type of variant and impact on protein, and on evidence from Clinvar and COSMIC databases. All results were discussed at a multi-disciplinary meeting. Other factors in determining whether a variant might be considered a driver mutation included tumour type, frequency of the variant in the tumour relative to tumour content analysed, and published literature regarding the variant in question. Data to support/refute pathogenicity of the same variant in the germline was also considered.
Phase I trial Allocation
All patients referred for consideration of a Phase I trials undergo initial clinical review to determine overall fitness to proceed based on current performance status, past medical history, medications and previous treatment. All cases are discussed at a weekly Patient Allocation Meeting, where this assessment, and other factors, such as tumour type, results of germline and somatic assessment and available trial slots are considered. Ideally, patients with confirmed pathogenic variants in the germline or soma are allocated to trials investigating utility of agents targeting the gene in question or a key pathway in which it is involved. In the case that no available trial exists, or if no slot is available on an existing trial, patients are allocated to the next best available trial depending on tumour type and patient factors. If rare variants of uncertain significance were identified in genes in which driver mutations were known to be associated with the tumour of interest, and if a trial using a targeted agent was available, cases were discussed with trial sponsors to determine patient eligibility.
Results
Patient Characteristics
Two hundred and nineteen patients diagnosed with cancer between the ages of 15 and 39 were referred to the Drug Development Unit in the Royal Marsden Hospital during the study period, including 139 (63%) females and 80 (37%) males. The median age of patients was 32 (15–39). The most common cancer type affecting these patients was sarcoma (n=41, 19%, Table 2), of which the most common subtype was Ewing (n=14, 34%, Table 3). Sarcoma was the most common cancer type in males (25, 31%), followed by colorectal (n=13, 16%), while the most common cancers in females in this cohort were cervical (n=27, 19%), breast (n=25, 18%) and ovarian (n=22, 16%, Table 2). Most patients had undergone at least one line of systemic therapy in the adjuvant setting before consideration of a phase I clinical trial (Table 4), but the majority had a relatively short interval between diagnosis and referral for consideration of a phase I agent (Median time to referral 29 months (1-237), Table 4).
Table 2. Cancer types in male and female AYAs.
| Cancer Type | N(%) | ||
|---|---|---|---|
| Female (n=139) | Male (n=80) | Total (n=219) | |
| Sarcoma | 16 (12) | 25 (31) | 41 (19) |
| Cervix | 27 (19) | 0 | 27 (12) |
| Breast | 25 (18) | 0 | 25 (11) |
| Ovarian | 22 (16) | 0 | 22 (10) |
| Colorectal | 8 (6) | 13 (16) | 21 (10) |
| Melanoma | 9 (6) | 7 (9) | 17 (8) |
| Brain | 6 (4) | 8 (10) | 14 (6) |
| Adrenocortical | 5 (4) | 2 (3) | 7 (3) |
| Cholangiocarcinoma | 3 (2) | 4 (5) | 7 (3) |
| Head and Neck | 2 (1) | 3 (4) | 5 (2) |
| Renal | 2 (1) | 3 (4) | 5 (2) |
| Germ cell | 1 (1) | 3 (4) | 4 (2) |
| HCC | 1 (1) | 3 (4) | 4 (2) |
| Lung | 3 (2) | 1 (1) | 4 (2) |
| MCUP | 1 (1) | 3 (4) | 4 (2) |
| Bladder | 1 (1) | 1 (1) | 2 (1) |
| Endometrial | 2 (1) | 0 | 2 (1) |
| Others | 4 (3) | 4 (5) | 8 (4) |
Table 3. Subtypes of sarcoma in male and female AYAs.
| Female | Male | All patients | |
|---|---|---|---|
| Ewing Sarcoma Family | 5 (31) | 10 (40) | 15 (37) |
| Malignant Peripheral Nerve Sheath tumour | 1 (6) | 3 (12) | 4 (10) |
| Osteosarcoma | 1 (6) | 3 (12) | 4 (10) |
| Desmoplastic small round cell tumour | 2 (13) | 1 (4) | 3 (7) |
| Alveolar soft part sarcoma | 1 (6) | 1 (4) | 2 (5) |
| Chondrosarcoma | 2 (13) | 0 | 2 (5) |
| Synovial Sarcoma | 2 (13) | 0 | 2 (5) |
| Clear Cell Sarcoma | 0 | 2 (8) | 2 (5) |
| Chordoma | 0 | 1 (4) | 1 (2) |
| Fibrosarcoma | 0 | 1 (4) | 1 (2) |
| Undifferentiated pleomorphic sarcoma | 0 | 1 (4) | 1 (2) |
| Pulmonary Sarcoma | 0 | 1 (4) | 1 (2) |
| Rhabdomyosarcoma | 0 | 1 (4) | 1 (2) |
| Leiomyosarcoma | 1 (6) | 0 | 1 (2) |
| Spindle cell sarcoma | 1 (6) | 0 | 1 (2) |
| 16 | 25 | 41 |
Table 4. Previous treatments and time to referral for different cancer types in AYAs.
| Median (Range) | ||
|---|---|---|
| Cancer Type | Number of previous lines of treatment | Length of time between diagnosis and referral to DDU (months) |
| Sarcoma | 2 (0-6) | 26 (2-164) |
| Cervical | 1 (0-3) | 22 (6-60) |
| Breast | 2 (1-5) | 52 (11-128) |
| Ovarian | 2.5 (0-6) | 31.5 (6-237) |
| Colorectal | 2 (1-4) | 23 (11-78) |
| Melanoma | 1 (0-3) | 43 (1-110) |
| Brain | 2 (0-3) | 55 (8-136) |
| Adrenocortical | 1 (1-2) | 20 (8-52) |
| Cholangiocarcinoma | 2 (1-3) | 17 (6-56) |
| Head and Neck | 1 (1-3) | 13 (10-32) |
| Renal | 2 (0-2) | 26 (17-47) |
| Germ Cell Tumour | 2 (2-3) | 99 (9-201) |
| Hepatocellular cancer | 2 (1-4) | 42.5 (5-151) |
| Lung | 2.5 (2-3) | 12.5 (11-21) |
| Bladder | 1.5 (0-3) | 19.5 (11-28) |
| Endometrial | 2.5 (2-3) | 21 (19-23) |
| Others | 1 (1-5) | 27.5 (13-70) |
| Metastatic Cancer of unknown Primary | 2 (1-3) | 11.5 (10-14) |
| All cases | 2 (0-6) | 29 (1-237) |
Family History of Cancer
In 81 (37%) individuals, no family history information was documented in either the patient’s referral documents or clinical notes. Twenty-four patients were noted to have a positive family history including a first degree relative with cancer. In ten cases, the positive family history was of a cancer with a potentially shared genetic aetiology. Forty-two patients had at least one second-degree relative with cancer, 19 of whom had a cancer with potentially shared genetic susceptibility. The family history of 40 individuals in this cohort was deemed “non-contributory” in the clinical notes, but no detail was given as to whether the information sought was limited to first degree relatives, or whether a history of other cancers was present in the family.
Germline Genetic Assessment
Thirty-four patients (16%) were documented to have had germline genetic testing, of whom 22 were reported to have pathogenic variants (Table 5). Pathogenic variants were most commonly reported in BRCA1 (n=15) or BRCA2 (n=3), in eight patients with ovarian cancer and ten patients with breast cancer (Table 6). In 12 cases, genetic reports were not available for review, as they had not been provided at the time of patient referral, and were not actively requested.
Table 5. Germline genetic testing in AYAs.
| N | Germline Genetic Assessment Undertaken | Positive Result/Clinical diagnosis | Eligible for germline genetic testing based on personal history only using current guidelines | |
|---|---|---|---|---|
| Sarcoma | 41 | 0 | 2 | n/a |
| Cervix | 27 | 0 | n/a | 0 |
| Breast | 25 | 14 | 12 | 12 |
| Ovarian | 22 | 13 | 8 | 8 |
| Colorectal | 21 | 2 | 1 | 0* |
| Melanoma | 17 | 0 | n/a | 0 |
| Brain | 14 | 0 | n/a | 0 |
| Adrenocortical | 7 | 0 | n/a | 7 |
| Cholangiocarcinoma | 7 | 0 | n/a | 0 |
| Head and Neck | 5 | 1 | 0 | 0 |
| Renal | 5 | 1 | 1 | 5 |
| Germ cell | 4 | 0 | n/a | 0 |
| Hepatocellular Carcinoma | 4 | 0 | n/a | 0 |
| Lung | 4 | 0 | n/a | 0 |
| Metastatic Cancer of Unknown Primary | 4 | 0 | n/a | 0 |
| Bladder | 2 | 0 | n/a | 0 |
| Endometrial | 2 | 1 | 0 | 0* |
| Others | 8 | 2 | 1 | 0 |
| Total | 219 | 34 (16%) | 25 (11%) | 32 (15%) |
| N | IHC analysis performed | Loss of ≥1 MMR protein | Additional patients eligible for IHC analysis | |
| Colorectal | 20 | 3 (15%) | 0 | 17 (85%) |
| Endometrial | 2 | 1 (50%) | 0 | 1 (50%) |
unless directed by IHC analysis
Table 6. Germline variants identified in AYAs.
| Gene | Pathogenic Variant | Number of patients | Cancer in patient carrying variant | |
|---|---|---|---|---|
| BRCA1 | c.427G>T | p.Glu143Ter | 1 | Breast |
| BRCA1 | c.1505_1509delTAAAG | p.Leu502Serfs | 1 | Ovarian |
| BRCA1 | c.3331_3334delCAAG | p.Gln1111fs | 2 | Breast (1) Ovarian (1) |
| BRCA1 | c.3756_3759delGTCT | p.Ser1253Argfs | 1 | Ovarian |
| BRCA1 | c.4327C>T | p.Arg1443Ter | 1 | Breast |
| BRCA1 | c.4574_4575delAA | p.Gln1525Argfs | 1 | Ovarian |
| BRCA1 | c.5266dupC_p.Gln1756ProfsX74) | p.Gln1756Profs | 1 | Breast |
| BRCA1 | c.5278-1G>T | p.? | 1 | Breast |
| BRCA1 | Duplication Exon 13 | p.? | 1 | Breast |
| PTEN | Unknown, no report available for review | 1 | Breast | |
| APC | Unknown, no report available for review | 2 | Colorectal (1) Desmoid (1) |
|
| VHL | Unknown, no report available for review | 1 | Kidney | |
| BRCA1 | Unknown, no report available for review | 5 | Ovarian (4) Breast (1) |
|
| BRCA2 | Unknown, no report available for review | 3 | Breast (3) | |
One variant of uncertain significance in BRCA2 (c.9205T>C; (p.Cys3069Arg)) was detected in a single patient diagnosed with hormone receptor and HER2-receptor-positive breast cancer at 23 years of age. It was not documented that TP53 analysis had been undertaken in this individual.
One patient with early-onset Triple Negative breast cancer had undergone predictive testing for a known familial BRCA2 mutation, in advance of her own diagnosis, but unfortunately was not referred for full diagnostic testing subsequently.
The majority of genetic testing was undertaken after formal consultation with a genetic specialist. Three patients had analysis of BRCA1 and BRCA2 as part of the mainstreaming genetic testing pathway(27, 28). Using current guidelines, an additional eighteen patients (10 breast, 8 non-mucinous ovarian) would now qualify for mainstreamed BRCA1/BRCA2 analysis in our institution. One of these individuals had been assessed by a clinical geneticist at the time of her diagnosis, but did not fulfil testing criteria at the time of her review, and died before criteria for testing were loosened.
Two other patients with breast cancer were reported to have inherited cancer predisposition syndromes, one with Cowden syndrome and another with type I Neurofibromatosis (NF1). Two other individuals with NF1 were included in this cohort, both of whom were diagnosed with malignant peripheral nerve sheath tumours. Two patients with Familial Adenomatous Polyposis (FAP) were also referred for consideration of Phase I trials, one of whom was diagnosed with colorectal cancer, and the other with a desmoid tumour. One patient with Von Hippel Lindau syndrome was assigned to a Phase I trial after developing metastatic clear cell cancer of the kidney. Uninformative germline testing of RET was undertaken in one patient with medullary thyroid cancer. MUTYH analysis was undertaken in one patient with early-onset mismatch repair-proficient colorectal cancer. Uninformative results were obtained from a multigene panel including CDKN1B, VHL, NF1 and MEN1 in a patient with thymic neuroendocrine tumour. One patient with early-onset endometrial cancer underwent formal genetic assessment, tumour immunohistochemical assessment of mismatch repair proteins (MMR IHC) and germline FH (Fumarate Hydratase) testing. No underlying genetic defect was identified. MMR IHC was also performed on the tumours of two patients with early-onset colorectal cancer, which was normal in both cases. No further genetic assessment was undertaken in these individuals. Using current guidelines (29), the seventeen other patients in this series with colorectal cancer that did not have any such investigations performed should have, at least, MMR IHC or microsatellite instability of their tumours to out-rule Lynch syndrome. In our centre, the other young patient with endometrial cancer would also have MMR IHC analysis under contemporary institutional practice (Table 5).
Three patients in this series had more than one primary cancer, including one individual with adrenocortical cancer aged 34 and secondary AML, one patient with breast cancer at 37 and previous history of Wilms’ tumour aged 12; neither of whom were referred for clinical genetics assessment, and one patient with metastatic olfactory aesthesioneuroblastoma with past medical history of Hodgkin’s lymphoma who was referred for genetics consultation, but died while awaiting an appointment.
Somatic Genetic assessment
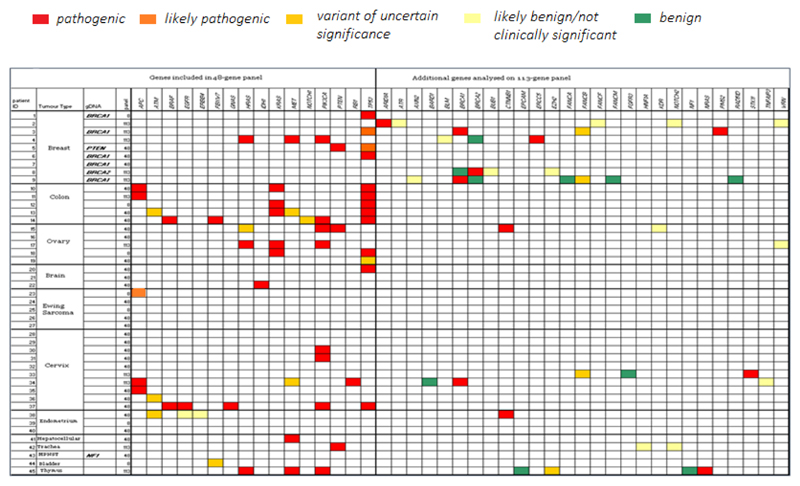
In thirteen tumours tested using the 48-gene panel, no somatic variants with allele frequency greater than 5% (major variants) were identified. At least one major variant least 5% was identified in 26 tumours (58%). Genes in which mutations were most commonly identified included TP53, KRAS, PIK3CA and MET (Figure 1). Increasing the panel size to 113-genes increased the yield of variants, but the majority of variants identified in genes that were not included on the 48-gene panel were either benign/passenger mutations, or pathogenic variants for which no targeted therapy currently exists.
Use of genomic profiling in trial allocation
Of eighteen patients with pathogenic BRCA1/BRCA2 variants, thirteen (72%) were allocated to a trial investigating utility of a Poly-ADP-ribose polymerase (PARP) inhibitor. Four other BRCA mutation carriers were allocated to trials investigating an agent involved in dsDNA damage response pathways. Considering all individuals (n=46) undergoing tumour molecular characterisation, nine were allocated to trials because of their underlying germline defect and seven assigned to trials of agents targeting identified somatic mutations or genes in a related pathway. Potentially actionable somatic mutations were identified in fifteen individuals, but at the time of their referral to the unit, there was either no suitable trial, or no slots available on a trial of interest. In three patients, allocation to a trial was not altered by tumour profiling, and assignation was on their tumour histology (Ewing sarcoma, n=2) or immunophenotype (HER2-positive breast cancer, n=1). In three patients, the crude number of mutations identified was considered a surrogate marker of high mutational load; and they were therefore assigned to trials of immunotherapy. For the other patients, no informative data were derived from germline or somatic genomic investigations.
Discussion
Cancer treatment in AYAs poses a unique set of challenges. Tumours in this cohort of individuals have been shown to demonstrate distinct biological behaviours compared to tumours of the same time occurring in the paediatric or older adult setting (9, 30–32). Furthermore, this cohort of individuals has unique psychosocial needs, and may have young children, siblings or parents for whom a familial risk of cancer may be relevant.
Recognition of a heritable cause of cancer is essential to provide optimal care to individuals with cancer. Identification of germline defects may modify therapeutic decisions(33); for example, germline mutations in BRCA1 or BRCA2 tend to confer sensitivity to Platinum-based chemotherapy(23), while identification of a TP53 germline mutation may alter surgical decision-making, or influence radiotherapy use or dose(34). In line with recent NICE guidelines (24), assessment of mismatch repair proteins by IHC is performed routinely in our institution on all colorectal cancers; and more recently, is also being routinely performed on all endometrial cancers. Identification of microsatellite instability or mismatch repair deficiency may help direct application of 5-FU based chemotherapy in Duke’s B colon cancer(35), and may also indicate the presence of a germline mutation causing Lynch syndrome.
In this cohort of unselected AYAs attending a phase I unit, investigations to confirm/out-rule an inherited cancer predisposition syndrome was, or would be undertaken using current guidelines in 89 (41%) patients, based on their personal cancer history alone. In this cohort, usually only a limited family history was recorded. In 81 (37%), no family history information was documented in any of the patient’s referral documents or clinical notes, and in an additional 40 patients, the family history was deemed “non-contributory”, but may not have expressly included a 3-generation history. It is likely therefore that genetic investigations would have been indicated in an even greater proportion if a detailed family history was taken into account.
In this particular cohort, the affected individuals had advanced cancer with poor prognoses. However, in AYAs with earlier stage cancer, the risk of second malignancies associated with a cancer predisposition syndrome is significant, and identification of a germline defect presents an opportunity to instigate prophylactic interventions. Furthermore, the significant risk to siblings, children and adults of these young individuals should also be considered, and preferably be discussed at an earlier stage of the disease). Consideration of a possible inherited germline defect in an individual in the palliative or experimental trial setting may provide the last opportunity for investigation in a family. In situations where an affected proband dies prior to genetic investigation, germline testing in the family is often impossible, as a negative result in an unaffected relative may not be reassuring to other individuals in the family. The results of this study suggest that germline genetic investigations should be routinely considered in AYAs with cancer, as the result may have a direct impact on management or allocation to a clinical trial.
Previous studies have shown that tumour-based genetic testing can inadvertently unmask a germline mutation in 3-5% cases(36, 37). In our cohort, pathogenic variants were identified in BRCA1 and BRCA2 in tumours of two individuals with breast cancer. These results were not unexpected, as in both cases, the patients had previously been known to carry germline mutations. It is likely that, as more tumour testing is undertaken using the 113-gene panel, more variants in cancer susceptibility genes will be identified. It is important, therefore, to bear the possibility of unmasking a germline event in mind, particularly if the patient is young, or with a positive family history suggesting an inherited cancer predisposition. At the present time, germline analysis to confirm constitutional status of a mutation in a particular gene in a patient without a positive family history may not be funded by the national health system (NHS), particularly if the mutation was identified in a tumour not known to be associated with germline mutations in that gene. To avoid this scenario, some units use germline DNA subtraction when interpreting their tumour sequencing results. This approach has the disadvantage of potentially missing targetable mutations. In our unit, and in most other oncology units, germline testing for the purpose of matched analysis is not routinely performed. All tumour results are discussed at a departmental clinical meeting. If any variant is suspected to be germline in origin, the case is discussed with the cancer genetics service, and germline testing undertaken, if appropriate. Due attention must be paid to the slight possibility of incidental results of this nature when consenting individuals for such testing, and concerns about how consent for such testing should be undertaken have been discussed within our unit and indeed internationally.
Interpretation and classification of somatic variants is extremely challenging. Where classification of germline variants is guided by stringent guidelines such as those of the American College of Medical Genetics (ACMG)(38) and Association for Clinical Genetic Science (ACGS) (39), no robust classification system exists for somatic variants. In tumours, pathogenicity is not an immediate indicator that the mutation is driving the neoplastic process. This must be considered when a variant is identified in a tumour in a gene in which mutations have not previously been reported to drive the specific cancer. Clinical utility of the test is an important factor to consider before offering the test, as identification of variants of uncertain significance, or variants in genes of uncertain significance may not be informative, but may generate significant anxiety and additional clinical work. Interpretation of somatic variation is further complicated by mutation frequency – a suspected driver mutation with low mutation frequency may represent a driver mutation in only a subclonal population of cells, or may be a late passenger event. Tumour heterogeneity adds complexity to interpretation of tumour testing, and factors such as site of the biopsy (central or peripheral; primary or metastasis), previous treatments and potential secondary mutational events should be considered.
Twelve patients were reported to have been identified as germline mutation carriers, but genetic reports had not been formally requested by our institution. We strongly recommend that genetic reports formally be reviewed, to confirm that the variant identified was pathogenic/likely pathogenic, and therefore clinically actionable. Genetic testing for NF1 is not routinely advocated, as this condition can be diagnosed on a clinical basis, and genetic testing is often uninformative if cDNA analysis has not been undertaken(40, 41).
The relative frequency of different cancer types in our cohort was biased by availability of different trials over the study trial; the cohort was enriched for Ewing sarcoma because of an ongoing IGF1-inhibitor trial; and for breast and ovarian cancers because of PARP-inhibitor studies.
Conclusion
Overall, 34 AYA patients (16%) did have germline genetic assessment, three (1%) had a confirmed genetic diagnosis, and two (1%) had tumour-based investigations. Using current testing criteria, an additional thirty-two (15%) of patients would qualify for a germline genetic test based on their personal history, and another 18 (8%) would have MMR IHC of their tumour. It is critical that heritable cancer predisposition be recognised in AYA patients in general, as it may have significant implications for the treatment and surveillance of the proband and his/her relatives. Ideally, diagnosis of a cancer predisposition syndrome should occur at an early stage in the patient’s treatment, to guide decision-making. The phase I setting is not an ideal scenario in which to discuss genetic testing and implications for the family. In this setting, patients will be discussing clinical trials and tumour molecular profiling, and discussing germline testing as well may be confusing or overwhelming. The potential impact to the wider family may also cause psychological in a vulnerable individual dealing with a life-limiting illness with little therapeutic options. Conversely, the phase I setting may provide one of the last opportunities to offer germline testing to these patients, and by extension, their families. Overall 40% of this cohort would have qualified for germline genetic testing using today’s criteria based on their personal history alone, and it is likely that a greater proportion still would also qualify if accurate family history information was recorded. Involvement of a cancer geneticist routinely in the multidisciplinary care of this unique young cohort would help optimise the overall treatment of the patient and their family, particularly in cases where the proband has limited life expectancy.
Acknowledgement
This is a summary of independent research supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and The Institute of Cancer Research. Dr McVeigh is also supported by the Health Research Board/Health Service Executive (HRB/HSE NSAFP/2014/1). The views expressed are those of the author and not necessarily those of the HRB/HSE, NHS, the NIHR or the Department of Health. None of the authors has a conflict of interest. The funding agency had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the paper; and in the decision to submit the paper for publication.
Footnotes
ECOG: Eastern Cooperative Oncology Group
Conflict of interest statement
The authors have no conflicts of interest to disclose.
References
- 1.Schmidt O. ABPI Guidelines for phase 1 clinical trials. 2012 [Google Scholar]
- 2.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–55. [PubMed] [Google Scholar]
- 3.Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14(8):463–482. doi: 10.1038/nrclinonc.2017.43. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez-Gastaldo A, Kempf E, González del Alba A, Duran I. Systemic treatment of renal cell cancer: A comprehensive review. Cancer Treatment Reviews. 2017;60:77–89. doi: 10.1016/j.ctrv.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Parakh S, Gan HK, Parslow AC, Burvenich IJG, Burgess AW, Scott AM. Evolution of anti-HER2 therapies for cancer treatment. Cancer Treatment Reviews. 2017;59:1–21. doi: 10.1016/j.ctrv.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite instability as a biomarker for PD-1 blockade. Clinical Cancer Research. 2016;22(4):813–820. doi: 10.1158/1078-0432.CCR-15-1678. [DOI] [PubMed] [Google Scholar]
- 8.W KA. DNA Sequencing Costs. Data from the NHGRI Genome Sequencing Program (GSP) 2017.
- 9.Zebrack B, Mathews-Bradshaw B, Siegel S. Quality Cancer Care for Adolescents and Young Adults: A Position Statement. Journal of Clinical Oncology. 2010;28(32):4862–4867. doi: 10.1200/JCO.2010.30.5417. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari A, Bleyer A. Participation of adolescents with cancer in clinical trials. Cancer Treatment Reviews. 2007;33(7):603–608. doi: 10.1016/j.ctrv.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Aben KK, van Gaal C, van Gils NA, van der Graaf WT, Zielhuis GA. Cancer in adolescents and young adults (15-29 years): a population-based study in the Netherlands 1989-2009. Acta Oncol. 2012;51(7):922–33. doi: 10.3109/0284186X.2012.705891. [DOI] [PubMed] [Google Scholar]
- 12.Fidler MM, Gupta S, Soerjomataram I, Ferlay J, Steliarova-Foucher E, Bray F. Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012: a population-based study. The Lancet Oncology. doi: 10.1016/S1470-2045(17)30677-0. [DOI] [PubMed] [Google Scholar]
- 13.Husson O, Lidington E, Younger E, van der Graaf WTA. Young adults: A unique group in cancer epidemiological research. Lancet. 2017 doi: 10.1016/S1470-2045(18)30029-9. in press. [DOI] [PubMed] [Google Scholar]
- 14.Herrmann LJM, Heinze B, Fassnacht M, Willenberg HS, Quinkler M, Reisch N, et al. TP53 Germline Mutations in Adult Patients with Adrenocortical Carcinoma. The Journal of Clinical Endocrinology & Metabolism. 2012;97(3):E476–E485. doi: 10.1210/jc.2011-1982. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez KD, Noltner KA, Buzin CH, Gu D, Wen-Fong CY, Nguyen VQ, et al. Beyond Li Fraumeni Syndrome: Clinical Characteristics of Families With p53 Germline Mutations. Journal of Clinical Oncology. 2009;27(8):1250–1256. doi: 10.1200/JCO.2008.16.6959. [DOI] [PubMed] [Google Scholar]
- 16.Varley JM, McGown G, Thorncroft M, James LA, Margison GP, Forster G, et al. Are there low-penetrance TP53 alleles? Evidence from childhood adrenocortical tumors. American Journal of Human Genetics. 1999;65(4):995–1006. doi: 10.1086/302575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner J, Portwine C, Rabin K, Leclerc JM, Narod SA, Malkin D. High frequency of germline p53 mutations in childhood adrenocortical cancer. Journal of the National Cancer Institute. 1994;86(22):1707–1710. doi: 10.1093/jnci/86.22.1707. [DOI] [PubMed] [Google Scholar]
- 18.Raymond VM, Else T, Everett JN, Long JM, Gruber SB, Hammer GD. Prevalence of Germline TP53 Mutations in a Prospective Series of Unselected Patients with Adrenocortical Carcinoma. The Journal of Clinical Endocrinology and Metabolism. 2013;98(1):E119–E125. doi: 10.1210/jc.2012-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogelaar IP, van der Post RS, Bisseling TM, van Krieken JHJM, Ligtenberg MJL, Hoogerbrugge N. Familial gastric cancer: Detection of a hereditary cause helps to understand its etiology. Hereditary Cancer in Clinical Practice. 2012;10(1) doi: 10.1186/1897-4287-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cisco RM, Norton JA. Hereditary diffuse gastric cancer: surgery, surveillance and unanswered questions. Future Oncol. 2008;4(4):553–9. doi: 10.2217/14796694.4.4.553. [DOI] [PubMed] [Google Scholar]
- 21.George A, Kaye S, Banerjee S. Delivering widespread BRCA testing and PARP inhibition to patients with ovarian cancer. Nature Reviews Clinical Oncology. 2017;14(5):284–296. doi: 10.1038/nrclinonc.2016.191. [DOI] [PubMed] [Google Scholar]
- 22.Krammer J, Pinker-Domenig K, Robson ME, Gönen M, Bernard-Davila B, Morris EA, et al. Breast cancer detection and tumor characteristics in BRCA1 and BRCA2 mutation carriers. Breast Cancer Research and Treatment. 2017;163(3):565–571. doi: 10.1007/s10549-017-4198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahman N. Realizing the promise of cancer predisposition genes. Nature. 2014;505(7483):302–8. doi: 10.1038/nature12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NICE. Molecular testing strategies for Lynch syndrome in people with colorectal cancer. 2017 [Google Scholar]
- 25.TGLclinical. TruSight Cancer - Methodology used for Cancer Predisposition Gene Tests. 2013 [Google Scholar]
- 26.TGLclinical. TGLclinical Services
- 27.Mainstreaming Cancer Genetics. 2017 [Google Scholar]
- 28.George A, Riddell D, Seal S, Talukdar S, Mahamdallie S, Ruark E, et al. Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients. Scientific Reports. 2016;6 doi: 10.1038/srep29506. 29506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McVeigh TP, Kerin MJ. Clinical use of the Oncotype DX genomic test to guide treatment decisions for patients with invasive breast cancer. Breast Cancer (Dove Med Press) 2017;9:393–400. doi: 10.2147/BCTT.S109847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bleyer A. Young adult oncology: the patients and their survival challenges. CA Cancer J Clin. 2007;57(4):242–55. doi: 10.3322/canjclin.57.4.242. [DOI] [PubMed] [Google Scholar]
- 31.Tai E, Pollack LA, Townsend J, Li J, Steele CB, Richardson LC. Differences in non-Hodgkin lymphoma survival between young adults and children. Arch Pediatr Adolesc Med. 2010;164(3):218–24. doi: 10.1001/archpediatrics.2010.4. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez CV, Barr RD. Adolescents and young adults with cancer: An orphaned population. Paediatrics and Child Health. 2006;11(2):103–106. doi: 10.1093/pch/11.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McVeigh T, George A. Personalisation of Therapy – clinical impact and relevance of genetic mutations in tumours. Cancer Research Frontiers. 2017;3(1):29–50. [Google Scholar]
- 34.Schon K, Tischkowitz M. Clinical implications of germline mutations in breast cancer: TP53. Breast Cancer Research and Treatment. 2017:1–7. doi: 10.1007/s10549-017-4531-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandala M, Cervantes A, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi64–72. doi: 10.1093/annonc/mdt354. [DOI] [PubMed] [Google Scholar]
- 36.Catenacci DVT, Amico AL, Nielsen SM, Geynisman DM, Rambo B, Carey GB, et al. Tumor genome analysis includes germline genome: Are we ready for surprises? International Journal of Cancer. 2015;136(7):1559–1567. doi: 10.1002/ijc.29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones S, Anagnostou V, Lytle K, Parpart-Li S, Nesselbush M, Riley DR, et al. Personalized genomic analyses for cancer mutation discovery and interpretation. Science Translational Medicine. 2015;7(283):283ra53–283ra53. doi: 10.1126/scitranslmed.aaa7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in medicine : official journal of the American College of Medical Genetics. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallis Y, Payne S, McAnulty C, Bodmer D, Sistermans E, Robertson K, et al. Practice Guidelines for the Evaluation of Pathogenicity and the Reporting of Sequence Variants in Clinical Molecular Genetics. 2013 [Google Scholar]
- 40.Evans DG, Bowers N, Burkitt-Wright E, Miles E, Garg S, Scott-Kitching V, et al. Comprehensive RNA Analysis of the NF1 Gene in Classically Affected NF1 Affected Individuals Meeting NIH Criteria has High Sensitivity and Mutation Negative Testing is Reassuring in Isolated Cases With Pigmentary Features Only. EBioMedicine. 2016;7:212–220. doi: 10.1016/j.ebiom.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferner RE, Huson SM, Thomas N, Moss C, Willshaw H, Evans DG, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. Journal of Medical Genetics. 2007;44(2):81–88. doi: 10.1136/jmg.2006.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]