Fig. 2.
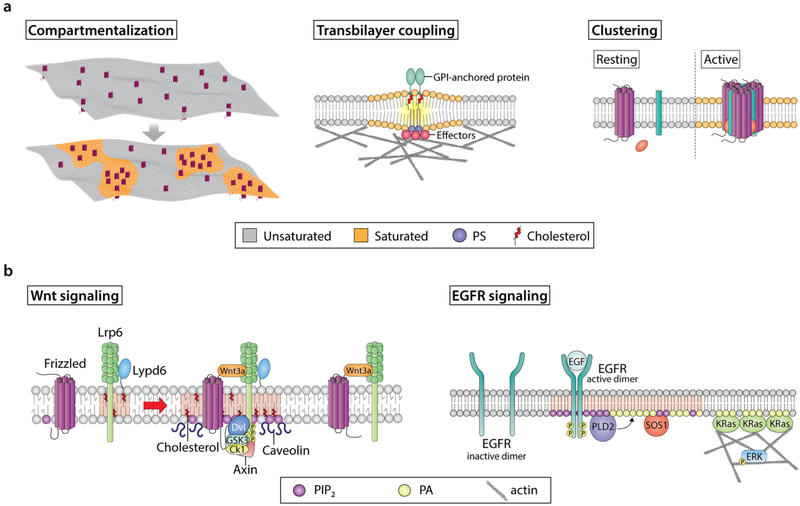
Modulation of lipid and protein organization in plasma membrane domains and their functions. a Examples of various membrane domain features. Highly dynamic interactions between lipid and protein molecules shape many of the features display by specialized plasma membrane domains. For example, the preferential interactions between specific proteins and cholesterol, sphingolipids, and, in multiple cases, charged signaling lipids can induce precise spatial compartmentalization of key membrane components, thus creating molecularly well-defined domains. This, in turn, regulates cellular signaling by mediating the recruitment of specific signaling effectors at an exact location and time. Another fundamental feature of membrane domains is proteolipid clustering. In many cases, assembly of clusters requires a stimulus to initiate the movement of cluster forming molecules between different membrane domains, resulting in the activation and oligomerization of these effectors. Plasma membrane clusters contain a variety of protein functionalities that can originate from both the plasma membrane and cytosol. Clustering of multiple functionalities at the membrane modulates high specificity and low membrane molecule diffusion, which in turn enhances signaling robustness. Lastly, proteins and lipids (e.g., cholesterol, GPI-anchored proteins, PS) are organized in plasma membrane domains within a bilayer with distinctive outer exoplasmic and inner cytoplasmic leaflets. These leaflets differ in terms of their lipid and protein compositions. Accordingly, specific membrane domains can induce the formation of proteolipid assemblies in the opposing leaflet. Inner leaflet effector organization is regulated by complex interactions between actin, lipids, and other proteins. These inner leaflet proteolipid assemblies can influence other effectors located in the outer leaflet and engage in transbilayer coupling. This is important, because transbilayer coupling is a mechanism by which membrane domain components are brought together at the two sides of the plasma membrane to efficiently signal. b Examples of the role of membrane domains in signaling events associated with cancer. Wnt signaling receptors, i.e., LRP6 and Fz, localize in both raft and non-raft domains. In the presence of Wnt, cholesterol bilayer asymmetry is triggered, which leads to enrichment of cholesterol in the inner leaflet. Moreover, Wnt-bound Fz-LRP6 complexes preferably localize to cholesterol-enriched membrane domains containing caveolin. This, in turn, leads to the recruitment of the Wnt signaling effector Dvl. The ability of Dvl to oligomerize promotes Fz and LRP6 clustering and recruitment of Axin, leading to LRP6 phosphorylation by GSK3 and CK1 in lipid rafts. Simultaneously, lipid kinases (e.g., PI4KII and PIP5KI, not shown) are recruited to these sites and promote production of PIP2, which in turn promotes LRP6 and Fz clustering and phosphorylation. Importantly, although Wnt-bound LRP6-Fz complexes can localize to non-raft domains, Lypd6, a GPI-anchored protein that localizes specifically in lipid rafts, ensures that LRP6 phosphorylation and thus receptor activation and efficient signaling occur in lipid raft domains. EGFR signaling is initiated from highly organized nanoscale proteolipid domains, driven by the spatiotemporal production of specific lipids. For example, EGFR activation by EGF initiates the formation of lipid domains, which are enriched in PIP2 and PA. The PA generated by PLD2 acts as a beacon to recruit KRas and SOS1, and along with PIP2, acts as a cofactor for SOS1-mediated activation of KRas. Furthermore, this pool of PA stabilizes the actin cytoskeleton, of which KRas nanoclustering is dependent. Together, these steps contribute to efficient ERK activation and downstream signaling