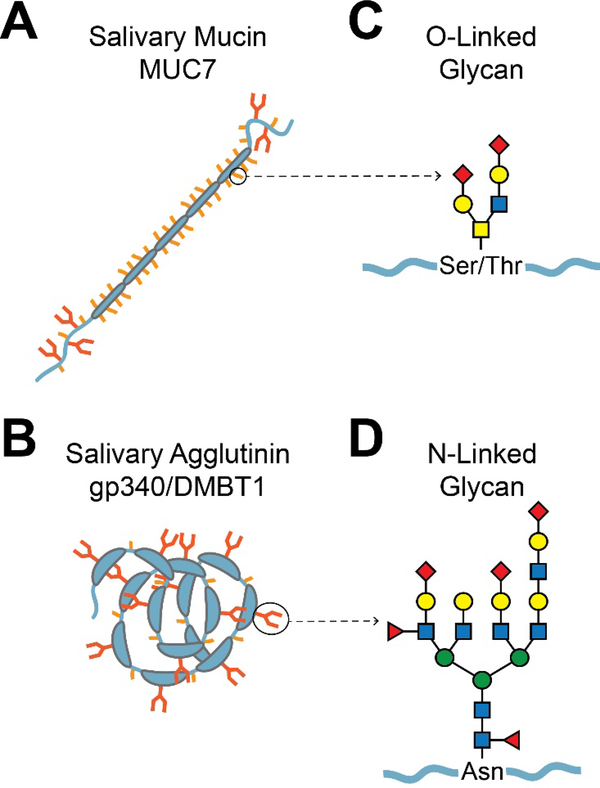
Fig. 2.
Typical salivary glycoproteins and their glycans. A. Mucins. Shown is a schematic diagram of the salivary mucin MUC7. The mucin protein backbone (apomucin) contains tandem repeats of proline, serine, and threonine-rich (PTS) domains, which are densely O-glycosylated. Non-repeat regions at the N- and C-terminals can be both O- and N-glycosylated. Dense O-glycosylation is believed to prevent typical protein folding, resulting in a bottle-brush-like shape. The larger gel-forming mucins such as MUC5B (not shown) are composed of several covalently linked mucin subunits. B. Agglutinins. Shown is a schematic diagram of the salivary agglutinin gp340/DMBT1. This protein contains several scavenger receptor cysteine-rich (SRCR) domains, which constitute the majority of the protein [263]. The SRCR domains often mediate ligand binding or protein-protein interactions with microbes. N-glycosylation occurs within the SRCR domains, and O-glycosylation occurs between the SRCR domains in serine-threonine-rich motifs that are approximately 20 amino acids in length [264]. C. Representation of a typical O-glycan chain. The base of an O-glycan is typically comprised of GalNAc α-linked to the hydroxyl group of serine or threonine. Other specific saccharide subunits and their attachments vary, but there are often two major branches with a small number of subunits. D. Representation of a typical Nglycan. N-glycans are attached to asparagine. All of the characterized eukaryotic N-glycans begin with a GlcNAcβ1-Asn linkage, but other saccharide linkages have been observed in prokaryotes. N-glycans are generally larger than O-glycans, containing more saccharide subunits and two to four major branches.