Abstract
In this review, we discuss the molecular mechanisms of cytokinesis from plants to humans, with a focus on contribution of membrane trafficking to cytokinesis. Selection of the division site in fungi, metazoans, and plants is reviewed, as well as the assembly and constriction of a contractile ring in fungi and metazoans. We also provide an introduction to exocytosis and endocytosis, and discuss how they contribute to successful cytokinesis in eukaryotic cells. The conservation in the coordination of membrane deposition and cytoskeleton during cytokinesis in fungi, metazoans, and plants is highlighted.
Keywords: Contractile ring, Cytokinesis, Endocytosis, Exocytosis, Membrane deposition
Overview of cytokinesis
Cell proliferation by cell division is essential for all organisms from bacteria to humans. Cytokinesis, the physical partition of one mother cell to create two daughter cells, is the final step of the cell division cycle. Cytokinesis requires the coordination of multiple cellular processes in order to produce two healthy and functioning cells (Storchova and Pellman 2004). Membrane trafficking and plasma membrane deposition at the division site contribute the materials to build a physical barrier between the two daughter cells, which is the ultimate purpose of cytokinesis.
Exocytosis delivers new membrane and cargoes to the plasma membrane during cytokinesis, cell growth, and cell motility. Endocytosis retrieves and recycles exocytic machinery for new rounds of exocytosis, and removes excess plasma membrane besides its other roles such as nutrient uptake. Thus, the coordination between exocytosis and endocytosis is essential for successful cytokinesis and other cellular events. Here, we review the basic machineries and recent progresses on cytokinesis, exocytosis, and endocytosis, with a focus on plasma membrane deposition and its coordination with the cytoskeleton in yeast, metazoan, and plant cells. Please refer to excellent recent reviews for comprehensive updates on other aspects of cytokinesis (D'Avino et al. 2015; Jürgens et al. 2015; Meitinger and Palani 2016; Rincon and Paoletti 2016; Bhavsar-Jog and Bi 2017; Pollard 2017; Basant and Glotzer 2018; Smertenko 2018).
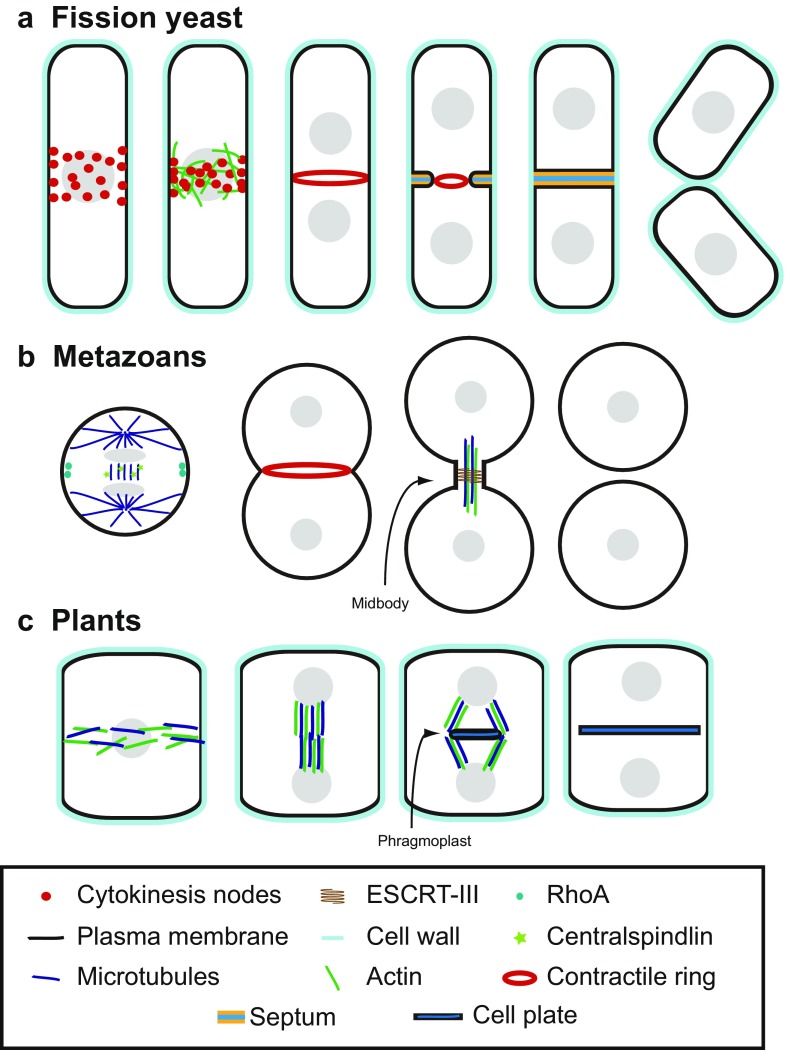
As the ability of cells to proliferate is a cornerstone of life, cytokinesis is well conserved across organisms. In coordination with cellular asymmetry, cytokinesis is also essential for cell differentiation by partitioning a mother cell into two daughter cells with different fates (Lacroix and Maddox 2012). There is some degree of variability on how cytokinesis is achieved for cells with various sizes and geometries (Moseley and Nurse 2010), but many of the homologous proteins and similar processes are widely used (Fig. 1). This allows insights learned in one organism to be often applied broadly to other organisms. Fungal cells, particularly the fission yeast Schizosaccharomyces pombe and the budding yeast Saccharomyces cerevisiae, have proved to be indispensable model systems for studying cytokinesis as they share a high degree of similarity with animal cells in the cell division cycle (Fig. 1a, b) while being genetically tractable, having efficient gene targeting by homologous recombination, and being ideal for microscopy (Balasubramanian et al. 2004; Rincon and Paoletti 2016). Lessons about the contractile-ring assembly, constriction, and function cannot be extended to plants due to their use of the expanding phragmoplast instead of a contractile ring for cytokinesis (Fig. 1c). However, the mechanisms related to membrane trafficking during cytokinesis might be widely conserved among plants, fungi, and metazoans.
Fig. 1.
Cytokinesis in different model organisms. a Cytokinesis in fission yeast. Cytokinesis nodes, containing myosin-II, mark the division site. Actin filaments promote node condensation to form the contractile ring by interaction with myosin-II. The ring begins to constrict after the cell exits from mitosis. Septum formation takes place behind the ring. The middle layer of the fully formed septum is then digested to separate the two daughter cells. b Cytokinesis in metazoans. Active RhoA promotes furrow ingression after being activated by centralspindlin. The actomyosin ring then forms at the division site. After furrow ingression, the secondary ingression at the midbody is mediated by the ESCRT-III complex. Abscission of the midbody leads to cell separation. c Cytokinesis in plants. Formation of the preprophase band marks the division site, made of actin and microtubules. The phragmoplast mediates the formation and expansion of the cell plate. Maturation and expansion of the cell plate completes the cell division by fusing with the plasma membrane
Failure in cytokinesis results in polyploidy or tetraploidy, which leads to cell death or oncogenesis in mammalian cells (Fujiwara et al. 2005; Ganem et al. 2007; Sagona and Stenmark 2010; Goyal et al. 2011). Premature cytokinesis damages the genome and leads to genomic instability as in the “cut” mutants in fission yeast (Yanagida 1998). In plants, failure to properly place the division site can lead to abnormal organogenesis (Smith et al. 1996). Six key events must be coordinated during cytokinesis in fungi and metazoans: (1) selection of the division site, (2) assembly of a contractile ring, (3) constriction and disassembly of the ring, (4) plasma membrane deposition/expansion, (5) extracellular matrix formation or remodeling, and (6) separation of the two daughter cells or abscission (Fig. 1a, b). Plants do not have either type II myosin or a contractile ring, but assemble a phragmoplast instead during cytokinesis (Fig. 1c). As a first step during cytokinesis, a site for division is selected at the cell equator or off center to have symmetrical or asymmetrical cell division, respectively. Then, a contractile ring of actin filaments, myosin-II, and at least two dozens of other proteins is assembled in fungal and animal cells during early mitosis or anaphase. The ring constriction guides or powers furrow ingression after mitotic exit. Targeted membrane deposition at the division site occurs before, during, and after ring constriction for the delivery of proteins and machinery that drive cytokinesis, as well as the membrane itself that is required to close the new end of the daughter cell. Along with plasma membrane deposition, remodeling and formation of the extracellular matrix, such as the septum in fungal cells, are also essential for successful cytokinesis in all cells (White and Bednarek 2003). Finally, the daughter cells physically separate from one another during abscission.
Molecular mechanisms of early events in cytokinesis
In this section, we briefly discuss the three early events during cytokinesis: division-site selection, contractile-ring assembly, and contractile-ring constriction.
Division-site selection and actomyosin contractile-ring assembly
The first step of cytokinesis is for the cell to select the division site in coordination with nuclear position or mitotic apparatus. To properly partition the genome, organelles, and cytoplasm of a mother cell, selection of the division site is critical in all systems. A landmark protein or protein complex is presumably placed at the selected site although the identities of this landmark are incompletely understood. Cells have adapted various ways to position proteins important for cell division at the cleavage site based on their specific needs.
The molecular mechanisms of division-site selection and contractile-ring assembly have been extensively studied in the fission yeast S. pombe (Pollard and Wu 2010; Lee et al. 2012; Rincon and Paoletti 2012; Willet et al. 2015). In S. pombe, the nucleus is maintained at the cell center by dynamic microtubule pushing against the cell cortex during interphase (Tran et al. 2001). Mid1, an anillin-like protein and the key player in division-site selection, is localized to cortical nodes on the plasma membrane and the nucleus during most of interphase (Bähler et al. 1998; Paoletti et al. 2000; Lee and Wu 2012). The Mid1 fraction on the plasma membrane partially depends on a cell size control pathway for localization (Rincon et al. 2014). This cell size control pathway seems to rely on the regulation of Wee1 kinase by interphase nodes containing kinases Cdr1 and Cdr2 (Martin and Berthelot-Grosjean 2009; Moseley et al. 2009). As the cell grows, these interphase nodes also accumulate and inhibit Wee1, which inhibits Cdk1 kinase (Guzman-Vendrell et al. 2015; Allard et al. 2018). So, when Cdr1/2 kinases inhibit Wee1, Cdk1 becomes active and promotes mitotic entry (Deng and Moseley 2013; Allard et al. 2018). Pom1, a kinase that localizes to the cell tips, also plays a role in division-site selection by preventing node formation near the cell tips (Bähler and Pringle 1998; Rincon et al. 2014). The nuclear portion of Mid1 is phosphorylated at G2/M transition and released by Plo1, a Polo kinase that localizes to the nucleus, mitotic spindle, and spindle pole bodies (Bähler et al. 1998). We found that Plo1 and putative Rho-GEF Gef2 have overlapping functions in Mid1 cortical localization (Ye et al. 2012). In summary, S. pombe selects the division site by a combination of positive signal from anillin Mid1 and negative cue from Pom1 kinase (Moseley and Nurse 2009) as the following: (1) proteins in interphase nodes recruit a fraction of Mid1 during interphase, (2) Pom1 excludes Mid1 from the cell tips, and (3) Polo kinase Plo1 and Rho-GEF Gef2 work together to release and recruit the remaining Mid1 from the nucleus to the cortical nodes during the G2/M transition.
Once on the plasma membrane and becoming active at the G2/M transition, Mid1 begins to recruit other proteins to the membrane to form cytokinesis nodes through two modules (Laporte et al. 2011; Padmanabhan et al. 2011). In one module, Mid1 recruits the IQGAP Rng2 and myosin essential light chain Cdc4, then myosin-II heavy chain Myo2 and regulatory light chain Rlc1. In another module, Mid1 recruits the F-BAR Cdc15. Both modules can recruit the formin Cdc12 (Laporte et al. 2011; Padmanabhan et al. 2011), which nucleates and assembles linear actin filaments from cytokinesis nodes (Coffman et al. 2013; Bestul et al. 2015; Zimmermann et al. 2017).
The dispersed cytokinesis nodes on the cortex over the nucleus condense together to form a contractile ring through dynamic interactions between actin filaments and myosin-II motors (Fig. 1a), which was illustrated by a model called search, capture, pull, and release (SCPR) (Vavylonis et al. 2008; Ojkic et al. 2011; Bidone et al. 2014). According to the SCPR model, actin filaments nucleated by the formin Cdc12 “search” on the cell cortex in random directions. If the filaments are within the capture radius of another node, it is “captured” by the myosin-II motor in that node. Next, the myosin “pulls” on the actin filament by walking toward its barbed end. After a short episode of movement, the actin filament is “released” from myosin by filament severing, turnovers of the formin or myosin from nodes, or myosin-II heads dissociate with actin filaments. Repeated cycles of SCPR with the help from actin crosslinking proteins slowly pulls the nodes closer to one another until they are all aligned at the cell equator into a contractile ring. The stochastic model can reliably reproduce ring formation in both 2D and 3D Monte Carlo simulations in about 10 min, which is the same amount of time observed in cells (Vavylonis et al. 2008; Bidone et al. 2014; Tamura et al. 2017).
Once a compact ring is assembled, it matures by recruiting more than a dozen other proteins during anaphase B (Wu et al. 2003). The architectures of cytokinesis nodes and the contractile ring have been solved by confocal and superresolution microscopy (Laporte et al. 2011; Laplante et al. 2016; Liu and Wu 2016; McDonald et al. 2017). The arrangement of proteins in the nodes renders myosin minifilaments dispensable for the contractile ring (Laplante et al. 2016; Liu and Wu 2016). It was shown that the contractile ring has roughly three layers: a proximal layer that contains membrane-bound proteins that anchor the ring and act as scaffolds, an intermediate layer that contains accessory proteins, and a distal layer that contains F-actin filaments, myosin motors, and actin cross-linkers (McDonald et al. 2017). These are the most detailed and comprehensive architectures of the contractile ring and its precursors in any model system.
In the budding yeast S. cerevisiae, the bud site selection determines the division site since the bud-mother neck is the future site for cytokinesis (Gladfelter et al. 2001). The bud site is selected in an axial pattern, a site adjacent to the old bud sites, or in a bipolar pattern, a site adjacent or opposite from the old bud sites (Chant and Pringle 1995). These bud sites are marked by Bud proteins that can be divided into three groups (Chant 1999). The first group of proteins contains Ras-related GTPase Rsr1, GAP Bud2, and GEF Bud5 that are essential for both budding patterns (Chant et al. 1991; Lee et al. 2015; Kang et al. 2018). The second group contains Cdc42-GEF Bud3, anillin Bud4, Axl1, and Axl2/Bud10 that are specific for the axial pattern (Chant and Herskowitz 1991; Roemer et al. 1996; Harkins et al. 2001; Kang et al. 2014; Wu et al. 2015). Bud3 and Bud4 are important for establishing axial budding pattern by recruiting Axl1 and Axl2. The third group contains Bud7, Bud8, Bud9, Rax1, and Rax2 that are specific for the bipolar pattern (Zahner et al. 1996; Kato et al. 2011). Bud8 marks the distal pole while Bud9 marks the proximal pole (Zahner et al. 1996; Schenkman et al. 2002). Another important regulator is the GAP protein Rga1 that inhibits Cdc42 at old bud sites to ensure these sites are not reused (Tong et al. 2007; Lee et al. 2015; Cheffings et al. 2016; Miller et al. 2017).
Septins are also critical for the division-site selection and contractile-ring assembly in budding yeast (Gladfelter et al. 2001). Septins are GTP binding proteins that can oligomerize with one another into filaments at the emerging bud site and bud neck (Bertin et al. 2008; Garcia 3rd et al. 2011; Glomb and Gronemeyer 2016). Cdc3, Cdc10, Cdc11, Cdc12, and Shs1/Sep7 are mitotic septins for cytokinesis (Byers and Goetsch 1976; Longtine et al. 1996; Faty et al. 2002). These septin proteins appear at the bud neck during bud emergence in early interphase where they serve as a scaffold for the proteins of the actomyosin ring (Douglas et al. 2005). They recruit type II myosin Myo1 to form a ring structure without actin filaments, followed by the formin proteins Bnr1 and Bni1, IQGAP Iqg1, and F-BAR protein Hof1 (Bi et al. 1998; Lippincott and Li 1998; Lippincott and Li 2000). Actin filaments nucleated by formins appear at the bud neck during anaphase to form a functional contractile ring together with myosin-II (Wloka and Bi 2012). The septins initially form a ring at the bud neck, followed by an hourglass structure, which then splits into two rings on either side of the contractile-ring before ring constriction (Lippincott et al. 2001; Vrabioiu and Mitchison 2006; Ong et al. 2014). Unlike budding yeast, septins are not involved in contractile-ring assembly in fission yeast and their roles in cytokinesis are still not well understood (Martín-Cuadrado et al. 2005; Zheng et al. 2018).
In metazoans, the division site is determined by signals from the mitotic spindle and astral microtubules, although the identity of the landmark proteins remains debated. The chromosomal passenger complex (CPC) and centralspindlin activate GTPase RhoA, through the GEF Ect2 near the central spindle (Chalamalasetty et al. 2006). Centralspindlin targets Ect2 to the medial cortex through Cyk4 to act as a positive signal for cytokinesis (Nishimura and Yonemura 2006). Inactivation of RhoA by Rho GAP throughout the cell confines RhoA to sites of Ect2 activation (Basant and Glotzer 2018). The astral microtubules of the spindle also play an important role in regulating RhoA by inhibiting its activation away from the cell equator. The manner in which the microtubules inhibit RhoA activation is still unclear, but may involve the regulation of the Ect2 GEF (Wagner and Glotzer 2016). RhoA is also important for targeting of anillin to the division site (Sun et al. 2015a). Thus, active RhoA, GEF Ect2, and/or anillin may serve as the landmark (Paolo D'Avino 2009). Once RhoA is activated, it will activate formin for the nucleation of actin filaments and promote myosin-II activation to promote ring formation (Bement et al. 2006; Goode and Eck 2007; Chen et al. 2017). Cortical actin flow may also contribute to contractile-ring assembly (Zhou et al. 2007; Chen et al. 2008; Salbreux et al. 2009). The process of ring assembly in metazoans is not as clear as in yeasts, but it is possible that a process similar to SCPR in fission yeast could be used as node-like structures have also been observed in metazoans (Maupin et al. 1994; Hickson and O'Farrell 2008; Lewellyn et al. 2011).
In most types of plant cells, the division-site selection relies on the preprophase band that helps to guide the placement of the phragmoplast as it is established (Rasmussen et al. 2013; Rasmussen and Bellinger 2018). Actin filaments and microtubules on the cell cortex form a preprophase band at early mitosis. The establishment of this band requires formins and kinesins which may crosslink actin and microtubules, and the TON1/TRM/PP2A (TTP) complex that contributes to protein phosphorylation at the band (Spinner et al. 2013). The TTP complex is composed of the protein phosphatase PP2A, an assembly helper TON1a and TON1b, and the TON1 recruiting motif proteins, TRMs. The preprophase band is disassembled in pro-metaphase after having recruited landmarks to mark the location of the division site. The nature of the landmark proteins is not entire clear, but may contain tangled and Air9 proteins, or the kinesins POK1 and POK2 (Walker et al. 2007; Rasmussen et al. 2011; Lipka et al. 2014; Martinez et al. 2017; Mir et al. 2018).
Although some proteins may differ, all these organisms use landmarks to indicate where the division site should be located and use positive and negative regulations to confine and stabilize this site. Further research is needed to address how similar metazoan ring assembly is to the processes observed in yeasts.
Actomyosin contractile-ring constriction
Once the contractile ring has been formed and matured at the selected division site, it must constrict to guide and/or power furrow ingression under the guidance of cell cycle signals such as the Hippo-like SIN and MEN pathways (McCollum and Gould 2001; Simanis 2015; Baro et al. 2017). Ring constriction is still an area of very active investigation due to uncertainty about the relative contribution of different forces (Cheffings et al. 2016). In all systems with a contractile ring, the ring will shed proteins as it constricts and become smaller in volume.
In fission yeast, several models have been proposed for actomyosin ring constriction. Stachowiak et al. proposed that a mechanism similar to the SCPR model with membrane anchored actin filaments and myosin-II motors is sufficient for ring constriction (Stachowiak et al. 2014). Computer simulations based on molecularly explicit 2D models that include features of the SCPR model explain the isometric tension of contractile rings in S. pombe protoplasts (Stachowiak et al. 2014), but do not take into account how ring constriction is coupled to membrane deposition and extracellular matrix remodeling in three dimensions. Another model relies on actin treadmilling, crosslinking, and processivity of the myosin (Oelz et al. 2015). However, some force calculations have predicted that the myosin-driven ring constriction produces a force too small to overcome the high turgor pressure in fission yeast cells (Proctor et al. 2012). This suggests that other pathways also contribute or coordinate with ring ingression. Therefore, some studies have suggested that construction of the septum behind the contractile ring helps furrow ingression (Proctor et al. 2012; Thiyagarajan et al. 2015). In fission yeast, the septum made of β-glucans and α-glucans is constructed at the same time as the ring constricts. The septum has three layers: a primary septum sandwiched with two secondary septa (Fig. 1a). After ring constriction and septum maturation, the primary septum is digested by glucanases to trigger daughter-cell separation (Baladrón et al. 2002; Dekker et al. 2004). The growth of the septum synthesized by glucan synthases during ring constriction could provide the main force for furrow ingression (Proctor et al. 2012). The ring has been shown to be anchored to the plasma membrane and also to the growing septum through protein interactions (Sun et al. 2015a). The paxillin Pxl1 helps mediate the interaction between β-glucan synthase Bgs1, which synthesizes and anchors to the primary septum, and the contractile ring (Cortés et al. 2016). The ring has also been shown to help guide septum formation by a curvature-sensitive mechanism to ensure even growth of the septum around the circumference to prevent an irregular septum shape that could lead to defects in furrow closure (Thiyagarajan et al. 2015; Zhou et al. 2015). Working together, the contractile-ring constriction and septum synthesis can drive successful furrow ingression in fission yeast.
In budding yeast, the myosin-II motor is not essential for ring closure (Bi et al. 1998; Lord et al. 2005) and a very different model has been proposed, which suggests that ring constriction is driven by depolymerization of actin filaments that are cross-linked to one another (Mendes Pinto et al. 2012). When the filament loses subunits, the cross-linkers detach and reattach, thereby creating a pulling force.
In metazoans, several factors are important for maintaining the furrow as it ingresses during ring constriction. RhoA, as discussed above, is important for promoting furrow ingression. Once ingression starts, anillin, septins, and MgcRacGAP are critical for stability of the furrow (Basant and Glotzer 2018). The actin cytoskeleton may help to push the daughter cells apart during furrow ingression which, along with adhesion to a surface, can drive furrow ingression even with reduced myosin activity at the contractile ring (Zang et al. 1997; Zhang and Robinson 2005). Several models have been proposed for how ring constriction proceeds in animal cells. Constriction could occur in a manner similar to the SCPR or actin depolymerization models discussed above (Zumdieck et al. 2007; Stachowiak et al. 2014). Additionally, a third model proposes that the ring is composed of contractile units that are removed from the ring as constriction progresses (Carvalho et al. 2009; Khaliullin et al. 2018). The fourth model has proposed that motors cause actin buckling which leads to filament shortening and constriction (Lenz et al. 2012). More work must be done to understand which of these models or combination of models best represents constriction in metazoans.
Plasma membrane deposition and extracellular matrix formation during cytokinesis
Membrane deposition and extracellular matrix formation/remodeling are essential for cytokinesis (Skop et al. 2004; McCusker and Kellogg 2012). While most of the proteins used for membrane deposition are well conserved, how exocytosis and endocytosis are coordinated with the contractile ring and other cytoskeleton during cytokinesis remains largely unknown. Endocytosis, in particular, is less well understood during cytokinesis because of poor spatiotemporal resolution in microscopy. As reviewed below, membrane deposition might be one of the most conserved aspects of cytokinesis in all eukaryotic cells.
Primer on exocytosis
Exocytosis delivers new membrane and cargoes to the plasma membrane from late Golgi, which is of great importance when one mother cell divides into two daughter cells. Building a physical barrier to partition cells requires the addition of membrane and the delivery of specific machinery. In general, trafficking of vesicles to the plasma membrane follows similar steps (Fig. 2a) (Whyte and Munro 2002; Zorec 2018). Vesicles can be delivered to the plasma membrane through random walk, but they are mostly transported by the microtubule and/or actin cytoskeleton (Bendezu et al. 2012; Noordstra and Akhmanova 2017; Papadopulos 2017). Once near the target membrane, the vesicle is “tethered” to the membrane by a vesicle tether. Then SNARE (Soluble NSF Attachment Protein Receptor) complexes form between SNARE proteins on the vesicle and on the plasma membrane. The assembled SNARE complex provides the force needed for membrane fusion (Whyte and Munro 2002). These basic processes are universal in fungal, plant, and animal cells (Cucu et al. 2017; Papadopulos 2017).
Fig. 2.
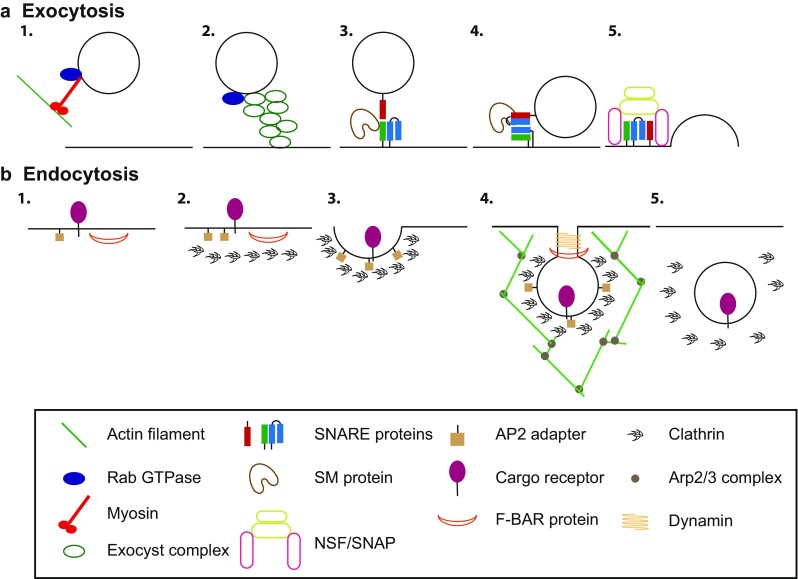
Illustration of exocytosis and endocytosis. a Exocytosis. (1) Vesicle delivery to the plasma membrane where it is (2) tethered by a tethering complex such as the exocyst, mediated by Rab GTPase. (3) SNARE proteins present on the vesicle and membrane are brought into proximity and (4) form a four helix bundle, templated by an SM protein. (5) SNARE complex formation drives fusion of vesicle to the plasma membrane, and NSF and SNAP proteins disassemble the cis SNARE complex. b Endocytosis. (1) Cargos and adaptor proteins on the membrane surface begin to cluster, (2) which recruits coat proteins such as clathrin. (3) The coat proteins promote membrane bending and invagination to form the endocytic pit. (4) Branched actin filaments are assembled and dynamin is recruited to the neck, with the help of F-BAR domain containing proteins, the vesicle is pinched off from the plasma membrane and is uncoated (5)
Rab GTPases are master regulators of exocytosis. They regulate delivery and fusion of vesicles (Pfeffer 2017). The delivered vesicles may also carry components of tethering complexes and SNARE proteins required for later steps in vesicle fusion (Jin et al. 2011). Once the vesicle is delivered to the target plasma membrane, it will be tethered before fusion. Tethering proteins enhance the efficiency of vesicle fusion and provide targeting specificity (Yu and Hughson 2010). Tethers include long coiled-coil proteins and multi-subunit tethering complexes, or MTCs (Dubuke and Munson 2016). The exocyst complex is a tether that is present on the plasma membrane and found in fungi, plants, and metazoans. It is an octomeric MTC composed of subunits Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84 (TerBush et al. 1996). A clear mechanism of how tethers improve fusion efficiency has not been established, but the exocyst components interact with many of the other factors involved in fusion of the vesicle that are discussed below, including SNAREs and SM proteins (Brunet and Sacher 2014). Much work has been done to elucidate the structure of the complex to better understand its interactions and how it performs its functions (Picco et al. 2017; Lepore et al. 2018; Mei et al. 2018). Other tethers have been demonstrated to work at the plasma membrane during cytokinesis, including Sro7, a tomosyn homolog, and the TRAPP-II complex, another MTC that normally regulates intra- and post-Golgi trafficking (Wang et al. 2016; Rossi et al. 2018). It is possible that multiple tethers provide specificity for vesicle targeting at different locations on the plasma membrane.
After vesicles have been tethered, the SNARE complexes can form to drive fusion. The SNARE proteins vary depending on the trafficking pathway, but are composed of t- and v-SNAREs indicating whether the SNARE is attached to the target or vesicle membrane. These SNAREs are usually homologs of syntaxin, SNAP-25, and VAMP (Yoon and Munson 2018). When the SNARE domains of these proteins interact, they form the SNARE complex with a four helix bundle. The syntaxin-like SNAREs can fold in a “closed” or an “open” conformation, with the closed one inhibiting SNARE complex formation (Yoon and Munson 2018). However, the SNARE complex alone is not sufficient to drive the fusion of vesicles to membranes or provide specificity for membrane targeting (Brandhorst et al. 2006; Wickner and Rizo 2017). Several other proteins are required to control this regulation and to increase overall fusion efficiency. The most important of these are Sec1/Munc18 proteins (SM proteins) and Munc13/Unc-13 proteins (Wickner and Rizo 2017). SM proteins may function as a template for the SNARE complex to form upon (Baker et al. 2015). SM proteins are well conserved in fungi, plants, and animals (Sec1 proteins in yeasts, Munc18 in mammalian cells, KEULE in plants). Munc13 is believed to be important for priming of vesicle fusion by changing syntaxin from its closed conformation to its open conformation (Guan et al. 2008; Ma et al. 2011; Yang et al. 2015). After vesicle fusion, the SNARE complex must be recycled to be used again. The proteins NSF and SNAP are used to break apart the four helix bundle and to recycle v-SNAREs to their proper locations (Ryu et al. 2015). The proteins involved in vesicle fusion are well conserved across systems and pathways, providing the core machinery for membrane trafficking in almost every situation.
Primer on endocytosis
Just as with exocytosis, endocytosis is well conserved throughout eukaryotes (Fig. 2b) (Wideman et al. 2014). When extracellular cargos bind to membrane receptors, these receptors begin to cluster together. The cytosolic side of these proteins begins to recruit coat proteins and clathrins that can induce membrane curvature. Alternatively, proteins bound to lipids on the cytosolic side of the plasma membrane or adaptor proteins will begin the recruitment of coat proteins. Then, the Arp2/3 complex is activated to nucleate branched actin filaments. The induced membrane curvature grows until it has formed a coated pit before scission separates the endocytic vesicle from the plasma membrane. Finally, the coat proteins and actin filaments are shed and the vesicle is directed to its destination. In this review, we will focus on clathrin-mediated endocytosis at it is the predominant pathway for internalizing cargos and membrane (Kaksonen and Roux 2018), but cells also utilize clathrin-independent mechanisms as well (Mayor et al. 2014).
In metazoans, initiation of the endocytic pit requires a number of different proteins on the plasma membrane. AP2 is an adaptor protein that helps clathrin accumulate at the plasma membrane (Kelly et al. 2014). Other proteins bind to specific lipids, often phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2], or cargo receptors to aid coat accumulation (Antonescu et al. 2011). The “F-BAR domain only protein 1 and 2” complex, or FCHO1/2, is required for endocytic pit formation and binds directly to the membrane (Henne et al. 2010). FCHO1/2 and AP2 then recruit scaffold proteins EPS15 (epidermal growth factor receptor substrate 15) and intersectins along with clathrin (Kaksonen and Roux 2018). In yeast, the markers for the formation of the pit are divided into early and late coat proteins, with proteins like Syp1, Ede1, Ent1/2, and Sla2 as the early coat and Pan1, End3, and Sla1 as the late coat proteins (Brach et al. 2014; Sun et al. 2015b). In both mammals and yeast, these proteins interact with each other, lipids, and clathrin to form the coat and begin pit formation. The amount of cargo recruited to the site must be regulated, as too little cargos would waste energy to internalize and too much cargos will interfere with the invagination process (Carroll et al. 2012).
Once the cargos and coat proteins are recruited, an endocytic pit begins to form by membrane bending. Two main processes aid in membrane bending and the invagination of the endocytic pit. First, clathrin helps induce membrane curvature. Second, networks of actin filaments aid clathrin in bending the membrane (Kukulski et al. 2012b). Branched actin networks nucleated by the Arp2/3 complex push the plasma membrane while the network is connected to the clathrin coat. In budding yeast, the proteins in the late phase of endocytosis link the Arp2/3 complex to the clathrin pit (Sun et al. 2015b). This helps push the invaginating vesicle away from the plasma membrane. This actin network is essential for endocytosis in yeasts due to very high turgor pressure (Dmitrieff and Nédélec 2015). In mammalian cells, membrane tension seems to dictate the importance of the actin networks, with actin becoming more essential at higher tensions (Boulant et al. 2011). Once the coated vesicle has invaginated, it separates from the plasma membrane in a process mediated by dynamin in the presence of GTP (Antonny et al. 2016). BAR proteins are also involved in the scission step and are believed to bend the membrane or sense membrane curvature (Daumke et al. 2014). One role suggested for these BAR proteins is to recruit dynamin to the endocytic neck. Other studies have suggested a more direct role for these proteins in promoting scission but this is still an open question to be investigated (Boucrot et al. 2012; Zhao et al. 2013).
Once the vesicle is separated from the plasma membrane and internalized, the clathrin coat must be removed before the vesicle can fuse with an internal organelle. The chaperone protein HSC70 and clathrin binding protein auxilin either bind to clathrin to weaken the clathrin-clathrin interactions or create collisions that weaken the coat structure and lead to the disassembly (Ungewickell 1985; Newmyer et al. 2003; Sousa and Lafer 2015). The dephosphorylation of PI(4,5)P2 lipid on the vesicle by synaptojanin also contributes to uncoating (Posor et al. 2015).
Plasma membrane deposition and extracellular matrix remodeling in metazoan cytokinesis
New plasma membrane is needed during cytokinesis in animal cells. For a thorough review of cellular trafficking in animal cells during cytokinesis, see Fremont and Echard (2018) (Fremont and Echard 2018). The prevailing model indicates that new membrane is inserted adjacent to the contractile ring at the leading edge of the cleavage furrows in metazoans (Bluemink and de Laat 1973; Shuster and Burgess 2002; Barr and Gruneberg 2007) and that the exocyst tethers the exocytic vesicles (Fig. 3a) (Barr and Gruneberg 2007; Neto et al. 2013). However, several studies suggested that membrane from vesicles is inserted in a broader zone during furrow ingression in large cells (Danilchik et al. 1998; Jesuthasan 1998; Feng et al. 2002; Danilchik et al. 2003; Danilchik and Brown 2008). Moreover, other studies suggested that plasma membrane is inserted at cell poles (Charras and Paluch 2008; Sedzinski et al. 2011). The ambiguity is partly resulted from the dearth of high spatiotemporal resolution tracking of highly dynamic exocytic and endocytic events during cytokinesis.
Fig. 3.
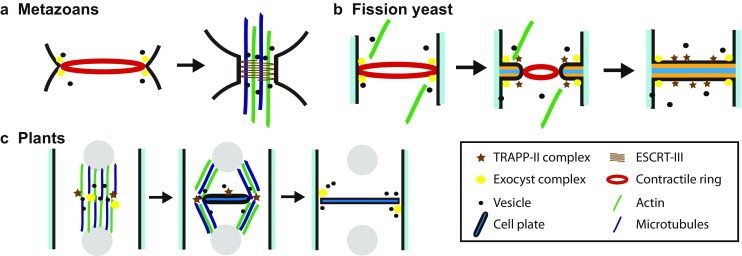
Membrane deposition and extracellular matrix formation at the division site during cytokinesis. a Animal cells. The exocyst localizes to the leading edge of the cleavage furrow just behind the contractile ring to tether vesicles during furrow ingression. At the midbody, actin and microtubules serve as tracks to deliver secretory vesicles and transport endocytic vesicles to promote abscission. b Fission yeast. Vesicles are initially tethered at the contractile ring by the exocyst tethering complex before ring constriction. The exocyst remains at the outer rim as the ring constricts and the TRAPP-II complex tethers vesicles along the division plane during septum formation. c Plant cells. Secretory vesicles are delivered to the phragmoplast by microtubule tracks and fuse together to expand outward to form the cell plate. The exocyst and TRAPP-II complexes cooperate in vesicle tethering and fusion
Vesicles from several origins arrive at the division site during cytokinesis. These include secretory vesicles from the late Golgi and different endosomal derived vesicles marked with various Rab GTPases. Once at the division site, the vesicles are tethered to the plasma membrane by the exocyst complex. The prevailing model would predict that localization of the vesicle tether, the exocyst complex, is guided by the contractile ring either physically or tension-sensitively. The exocyst is believed to be localized to the leading edge of the cleavage furrow (Fielding et al. 2005). It plays a critical role in vesicle fusion as discussed above, from tethering of the vesicle, to interacting with and activating the SNARE complex (Yue et al. 2017). It is unknown if other vesicle tethers are involved in membrane deposition at the cleavage furrow. Interestingly, it has been shown that TRAPP-II is important for furrow ingression in Drosophila cells (Robinett et al. 2009) and MICAL3 tethers vesicles from the endosome (Liu et al. 2016a). More research will help further untangle what cargos are carried on which vesicles and how they are targeted to specific locations at the division site. Rab GTPases are critical for regulation of vesicle trafficking in animal cells. Rab8, Rab11, and Rab35 all play important roles in the targeting and fusion of vesicles (Stenmark 2009). These proteins regulate secretion and recycling of proteins and membrane through the endosomal trafficking, which contributes greatly to furrow ingression (Gerald et al. 2001; Feng et al. 2002). As the furrow ingresses, the coordination of exocytosis and endocytosis is of great importance to expand the plasma membrane (Prekeris and Gould 2008). Membrane from recycling endosomes can be targeted back to the furrow and help to mitigate the loss of membrane, allowing cells to redistribute membrane and cargos to new sites.
Vesicles are known to be delivered to the cleavage furrow from both daughter cells during contractile-ring constriction (Fig. 3a) (Albertson et al. 2008). After ring constriction, two daughter cells are connected by an electron dense structure called midbody. When the cell transitions from furrow ingression to the secondary ingression at the midbody, endocytosis becomes more dominant as the cell removes actin and microtubules from the midbody (Schiel et al. 2012). Secretory vesicles are still seen in the midbody, and these may be important for delivering proteins needed for abscission (Goss and Toomre 2008). ESCRT-III is central to secondary ingression and is believed to help constrict the membrane to aid the closure of this site (Addi et al. 2018). Rab35 and Rab11 marked endosomes are important for the removal of actin filaments from the midbody. Factors such as OCRL, p50RhoGAP, and MICAL1 help depolymerize actin filaments to clear them (Dambournet et al. 2011; Klinkert and Echard 2016). This promotes the function of ESCRT-III during the secondary ingression and abscission. Tension in the membrane has also been shown to aid abscission. This tension relies on integrin proteins, and cells that do not have adequate tension are unable to resolve the midbody (Gupta et al. 2018). In summary, what we know about membrane deposition during cytokinesis in metazoans is still limited, especially on the coordination between the contractile-ring constriction and plasma membrane expansion.
In addition to plasma membrane deposition, extracellular matrix remodeling is also essential for metazoan cytokinesis. Chondroitin sulfate proteoglycans are involved in cytokinesis (Izumikawa et al. 2010; Izumikawa et al. 2014). They are made of a core protein with a sugar side chain and are secreted from cells. Interestingly, loss of the core proteins in both worms and mice results in embryo death due to failure in cytokinesis (Mizuguchi et al. 2003; Olson et al. 2006; Izumikawa et al. 2010). The role of chondroitin sulfate proteoglycans in cytokinesis has not been sufficiently studied in post-embryonic cells, however. In both mice and worms, a large secreted protein named hemicentin is important for stabilizing and promoting furrow ingression. In Caenorhabditis elegans germ cells and mouse embryos, hemicentin accumulates around the cleavage furrow, and its loss leads to furrow ingression failure (Xu and Vogel 2011). However, hemicentin is not needed for cell division in other cell types in worms and it is still unclear if it is used in cell division in the developed mouse.
Plasma membrane deposition and septum formation in yeast cytokinesis
Budding yeast is pivotal in revealing the molecular mechanisms of exocytosis and endocytosis (Goode et al. 2015; Zeng et al. 2017). However, membrane trafficking during cytokinesis is less understood due to the crowdedness of events at the bud neck. As in metazoans, new plasma membrane must be added during yeast cytokinesis. The amount of membrane needed to close the division site is cell type/geometry dependent. In the fission yeast S. pombe, it is estimated that net membrane from > 600 vesicles must be added to build the plasma membrane for two new daughter cells (~ 3.5 μm in diameter) during ring constriction that takes ~ 30 min, without considering membrane loss from endocytosis (Wang et al. 2016). In the budding yeast S. cerevisiae, membrane from > 50 vesicles is needed to fill in the ~ 1-μm bud neck. This membrane may come through the exocytic pathway because in secretory mutants, ring constriction and membrane invagination slows significantly (Jäntti et al. 2002; Wang et al. 2002). In addition, recycling endosomes from endocytosis may also contribute new membrane. S. pombe cells have ~ 100 endocytic actin patches, sites of endocytosis, at the division site during cytokinesis (Berro and Pollard 2014). These patches turn over in ~ 20 s and produce a vesicle with a surface area of ~ 0.0064 μm2 (Kukulski et al. 2012a; Berro and Pollard 2014). As a result, ~ 100 secretory vesicles are needed per minute at the division site to expand the membrane during ring constriction, far exceeding the ~ 20 net vesicles without considering endocytosis. It is possible that some lipids may be exchanged through contact sites between the ER and the plasma membrane (Wu et al. 2018). However, how much membrane these sites might contribute during cytokinesis is unclear.
In fission yeast, the exocyst tethering complex is localized to the outer rim of the division site before, during, and after ring constriction (Wang et al. 2002; Martín-Cuadrado et al. 2005; Bendezu and Martin 2011; Jourdain et al. 2012). It is separated from the contractile ring during ring constriction, which suggested that vesicles were tethered at the rim by the exocyst or at the leading edge of the cleavage furrow by another tether if the prevailing model in metazoans is applicable in fission yeast. Surprisingly, neither idea is completely correct (Wang et al. 2016). It was found that vesicles are deposited evenly along the cleavage furrow. Vesicles are deposited at the rim before ring constriction. During ring constriction, additional tethers are used to increase the specificity of vesicle delivery. The TRAPP-II complex may tether vesicles in addition to the exocyst complex in S. pombe (Wang et al. 2016). TRAPP-II appears to localize throughout the division plane, distinct from the outer rim localization of the exocyst complex. This suggests these tethers can coordinate and provide additional specificity for targeting vesicles to certain areas of the division site. It is possible that the exocyst is the dominant tether at the start of furrow ingression and at cell separation, while TRAPP-II is dominant during ring constriction and septum maturation (Fig. 3b). This would seem similar to the role of TRAPP-II during cytokinesis as described below in plants (Rybak et al. 2014).
The early delivery of vesicles before ring constriction may deliver important cargos needed for ring constriction. Before ring constriction begins, many transmembrane proteins vital for septum formation are delivered to the ring and presumably tethered by the exocyst in fission yeast (Muñoz et al. 2013; Arasada and Pollard 2014; Wang et al. 2016). Bgs1, Bgs4, and Ags1 are the key glucan synthases present in fission yeast that help to construct the primary and secondary septum. Recruitment of Bgs1 depends on the F-BAR protein Cdc15 as well as Sbg1 (Arasada and Pollard 2014; Davidson et al. 2016). Bgs4 helps connect the contractile ring to the cell wall to keep the ring from sliding (Muñoz et al. 2013). Bgs4 localization and dynamics are partially regulated by the Rho GAP Rga7 and the coiled-coil protein Rng10 (Arasada and Pollard 2015; Liu et al. 2016b). In yeasts, construction of the septum behind the contractile ring has been suggested to drive furrow ingression and produce the major force for cytokinesis (Proctor et al. 2012). For the daughter cells to separate at the end of cytokinesis, the primary septum must be digested to break apart the cells. Secretion of the Agn1 and Eng1 glucanases is the key to this digestion. The vesicles containing the enzymes might be mainly tethered by the exocyst complex at the rim of the division site (Martín-Cuadrado et al. 2005). Rho4 GTPase has been shown to regulate this secretion through the exocyst and septins (Pérez et al. 2015; Wang et al. 2015).
Exocytosis must coordinate with endocytosis during cytokinesis to expand the plasma membrane. Endocytosis during cytokinesis was rarely studied due to poor spatial resolution. Endocytosis recycles machinery for exocytosis and extra proteins from the plasma membrane during cytokinesis. Thus, the coordination between exocytosis and endocytosis is essential, but it is poorly understood. Ync13, an Unc-13/Munc13 protein, plays a role in organizing exocytosis and endocytosis during cytokinesis (Zhu et al. 2018). Loss of Ync13 dramatically affects endocytic sites on the division plane (Zhu et al. 2018).
In budding yeast, the leading edge of the cleavage furrow has been proposed to be the sites for membrane deposition (VerPlank and Li 2005; Wloka and Bi 2012) and the exocyst is the best known vesicle tether on the plasma membrane (VerPlank and Li 2005; Barr and Gruneberg 2007; Luo et al. 2014). Membrane delivery is targeted to the daughter cell for bud growth during interphase. The septin ring that forms at the bud neck was proposed to create a barrier between the plasma membrane in the mother and daughter cells (Takizawa et al. 2000; Dobbelaere and Barral 2004). Septins scaffold the contractile ring during its assembly and splits into two rings on both sides of the actomyosin ring before the ring begins constriction (Lippincott et al. 2001). It has been suggested the septins play an important role in confining other proteins to the division site, such as the exocyst complex as diffusion barrier since the exocyst subunit Sec3 localizes between the split septin ring, but not on the septin rings (Dobbelaere and Barral 2004). Thus, septins may confine the exocyst complex and the vesicles it tethers to a defined area of the division site just behind the contractile ring for membrane deposition. This barrier confines an active Cdc42 zone to the growing daughter cell to direct polarized exocytosis (Okada et al. 2013). However, the barrier role of septins during cytokinesis is still debated as in septin mutants that do not form double rings at the division site, cytokinesis proceeds efficiently, and all proteins needed for the ring, membrane trafficking, and cell wall synthesis are recruited normally (Wloka et al. 2011). Sro7 has also been shown to act as a tether at the division site in yeast, interacting with Sec4 GTPase (Rossi et al. 2018). Thus, while the exocyst seems to be the dominant tether at the plasma membrane, some other proteins are also present and contribute to vesicle fusion at the bud neck.
Budding yeast also constructs a septum made of chitin and glucans in a three-layer structure with primary and secondary septa. Chitin synthase 2 (Chs2) is responsible for the construction of the primary septum during ring constriction (Sburlati and Cabib 1986; Cabib 2004). The secondary septum is synthesized by glucan synthases Fks1 and Fks2 and chitin synthase Chs3 (Bhavsar-Jog and Bi 2017). Failing to deliver Chs2 to the bud neck prevents the contractile ring from constricting, and this delivery is mediated by the secretory pathway and the exocyst complex (VerPlank and Li 2005). Additionally, ingression progression complexes (or IPCs) mediate a connection between the contractile ring and septum synthesis. These complexes contain myosin-II Myo1, IQGAP Iqg1, Chs2, F-BAR Hof1, C2 domain protein Inn1, and transglutaminase Cyk3 (Foltman et al. 2016). The cell polarity protein Spa2 is needed for Chs2 to be incorporated into the IPCs (Foltman et al. 2018). Hof1 is an important inhibitor of Chs3 to ensure that secondary septum formation does not begin until the ring has constricted and primary septum has been formed (Oh et al. 2017). Rho1 is a critical regulator of extracellular matrix remodeling, as it controls the activities of Fsk1 and Chs3, as well as regulating the localization of the exocyst complex (Qadota et al. 1996; Guo et al. 2001; Valdivia and Schekman 2003). Endocytosis plays an important role in regulating septum formation during cytokinesis in budding yeast. Endocytosis was shown to retrieve chitin synthases Chs2 and Chs3 from the division site (Chuang and Schekman 1996). Internalization of Chs2 is performed by clathrin-mediated endocytosis; disruption of the retrieval by endocytosis mutants led to an increase in septum formation and constriction rate (Chin et al. 2016). Accumulation of cell wall integrity sensors Wsc1 and Wsc2 in endocytosis mutants also indicates that endocytosis at the bud neck is important to prevent abnormal cell wall remodeling (Wilk et al. 2010).
Collectively, yeasts have proven to be highly successful model systems for studying membrane deposition and septum formation during cytokinesis. Many of the discoveries made in yeasts are conserved in metazoans. However, much more work still must be done to reveal to the coordination of contractile ring, exocytosis, and endocytosis during cytokinesis.
Plasma membrane deposition and cell plate formation in plant cytokinesis
Plasma membrane deposition at the division site in plant cells shares many similarities with animal and fungal cells despite their lack of a contractile ring (Fig. 3c). The phragmoplast is a membrane structure associated with microtubules and actin filaments that guides the formation of the cell plate between two daughter cells. As the phragmoplast expands outward from cell center, the cell plate is formed centripetally behind it in the tubulovesicular structure created by the phragmoplast. Eventually, the membrane and cell plate fuse with the plasma membrane and side wall to form a new cell wall (Fig. 3c). During the expansion, vesicles are delivered via the phragmoplast microtubules and actin filaments (Staehelin and Hepler 1996). In contrast with much smaller yeast cells, approximately 95,000 vesicles must fuse at the membrane network at the phragmoplast in meristematic cells of Arabidopsis (Segui-Simarro and Staehelin 2006). Microtubules direct the delivery of vesicles toward the leading edge of the expanding phragmoplast and are stabilized by the cross-linker MAP65-1a (Murata et al. 2013). The homotypic fusion of the vesicles expands outward until it seals the two daughter cells. This method of vesicle delivery and fusion requires different regulation than the heterotypic fusion in other systems, in that t-SNARE and v-SNARE proteins are present on both membranes as they are derived from the same source. Later in cytokinesis, as the phragmoplast expands, fusion may become more similar to heterotypic fusion seen in other systems.
Recent work has shown that vesicles are delivered in three stages during cell plate formation (van Oostende-Triplet et al. 2017). First, there is a rapid delivery of vesicles without cell plate expansion. This is followed by phragmoplast expansion with more rapid vesicle delivery and cell plate expansion. Lastly, a slow phase ensues when the cell plate and membrane approach the marked region previously occupied by the preprophase band for fusion. The components needed for the fusion of these vesicles are very similar to those used in animal cells and fungi. The exocyst complex tethers vesicles before fusion just as in animals and yeast, but the TRAPP-II complex also aids tethering (Jaber et al. 2010; Qi et al. 2011). This beautiful work showed that the exocyst and TRAPP-II cooperate during the initial formation of the phragmoplast, and then TRAPP-II becomes the dominant tether during phragmoplast expansion. The exocyst is again needed for maturation of the phragmoplast (Rybak et al. 2014). A cytokinesis-specific syntaxin-like SNARE named KNOLLE is used to help target vesicles to the cell plate (Lauber et al. 1997). KEULE is a cytokinesis-specific SM protein that interacts with KNOLLE to convert it into the open conformation to allow SNARE complex assembly (Karnahl et al. 2018). KNOLLE specifically marks the phragmoplast and is removed from the membrane by endocytosis once the cell plate has fused with the membrane (Boutté et al. 2010). Thus, many of the proteins used for exocytosis are well conserved in cytokinesis.
Since the phragmoplast is constructed and expands from the center outward, the cell must control this growth to properly place the division site. As the phragmoplast expands toward the outer plasma membrane, expansion is directed and organized to prevent a misplaced division plane. This is controlled through actin and microtubule networks. Mutations in myosin VIII and class XII kinesins compromise the placement of the cell plate (Lipka et al. 2014; Wu and Bezanilla 2014). Microtubule binding proteins TAN1 and AIR9 are also critical for phragmoplast placement, indicating the microtubule network helps to organize and direct the growth of the phragmoplast (Mir et al. 2018). The role of actin has been less studied, but myosin VIII has been shown to localize to the leading edge of the phragmoplast and at the cell cortex to guide expansion toward the division site previously marked by preprophase band. Formin For2A nucleates actin filaments from the phragmoplast leading edge to provide tracks for myosin VIII, suggesting a role of actomyosin cytoskeleton in phragmoplast guidance (Wu and Bezanilla 2014).
Clathrin localizes to the spindle and the phragmoplast during its expansion, followed by concentration at the cell plate as it forms at the location of phragmoplast fusion with the plasma membrane (Tahara et al. 2007; Van Damme et al. 2011). In addition to clathrin, an adaptor protein TPLATE also localizes to the former location of the preprophase band (Van Damme et al. 2011). It was suggested that these proteins could be driving endocytosis to remodel the membrane in preparation for fusion to the phragmoplast or to control and confine KNOLLE to the division site. The role of endocytosis at this late stage of cytokinesis is supported by experiments showing inhibition of endocytosis has a strong negative effect on the slow final phase of cell plate expansion (van Oostende-Triplet et al. 2017).
Conclusion and future directions
While at first glance cytokinesis in plants appears quite different from metazoans and yeasts, they may share more similarities than differences. While there is no contractile ring in plants, the proteins and processes involved in membrane deposition seem to be well conserved across eukaryotes. Exocytosis contributes membrane to the expanding cleavage furrow or phragmoplast, while also delivering key membrane proteins for cytokinesis. This is balanced with endocytosis that regulates protein concentrations and membrane growth. Remodeling of the extracellular matrix has also been demonstrated to aid cytokinesis in very diverse organisms. In the last several decades, we have made rapid progresses on understanding molecular mechanisms of the key events in cytokinesis, and have discovered most of the important proteins involved in various steps. However, we still know very little on the coordination of all the events and processes during cytokinesis. There are still many burning questions to be answered. How does the contractile-ring constriction guide and coordinate with exocytosis and endocytosis? Does membrane tension regulate the balance of membrane addition and removal? How much do the vesicle tethers convey specificity for vesicle fusion? Are vesicles with distinct cargo composition specifically targeted to different locations along the division plane using different tethers? How conserved are the contributions of extracellular structures to cytokinesis? These and many other open-ended questions should be tested in multiple systems to unravel the conserved mechanisms of cytokinesis and variations on a theme.
Acknowledgments
We are grateful to Tom Pollard for all the mentoring and support of our studies on cytokinesis. We thank Yajun Liu, Larissa Valle Guilhen Longo, Rongkai Guo, and Sha Zhang for critical reading of the manuscript.
Funding
This work was supported by the grant R01GM118746 from the National Institute of General Medical Sciences of NIH to JQW.
Conflict of interest
Kenneth S. Gerien declares that he has no conflict of interest. Jian-qiu Wu declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Addi C, Bai J, Echard A. Actin, microtubule, septin and ESCRT filament remodeling during late steps of cytokinesis. Curr Opin Cell Biol. 2018;50:27–34. doi: 10.1016/j.ceb.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Albertson R, Cao J, T-s H, Sullivan W. Vesicles and actin are targeted to the cleavage furrow via furrow microtubules and the central spindle. J Cell Biol. 2008;181:777–790. doi: 10.1083/jcb.200803096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard CAH, Opalko HE, Liu K-W, Medoh U, Moseley JB. Cell size-dependent regulation of Wee1 localization by Cdr2 cortical nodes. J Cell Biol. 2018;217:1589–1599. doi: 10.1083/jcb.201709171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonescu CN, Aguet F, Danuser G, Schmid SL, Gruenberg JE. Phosphatidylinositol-(4,5)-bisphosphate regulates clathrin-coated pit initiation, stabilization, and size. Mol Biol Cell. 2011;22:2588–2600. doi: 10.1091/mbc.e11-04-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonny B, et al. Membrane fission by dynamin: what we know and what we need to know. EMBO J. 2016;35:2270–2284. doi: 10.15252/embj.201694613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasada R, Pollard TD. Contractile ring stability in S. pombe depends on F-BAR protein Cdc15p and Bgs1p transport from the Golgi complex. Cell Rep. 2014;8:1533–1544. doi: 10.1016/j.celrep.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasada R, Pollard TD. A role for F-BAR protein Rga7p during cytokinesis in S. pombe. J Cell Sci. 2015;128:2259–2268. doi: 10.1242/jcs.162974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler J, Pringle JR. Pom1p, a fission yeast protein kinase that provides positional information for both polarized growth and cytokinesis. Genes Dev. 1998;12:1356–1370. doi: 10.1101/gad.12.9.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler J, Steever AB, Wheatley S, Wang Y-L, Pringle JR, Gould KL, McCollum D. Role of polo kinase and Mid1p in determining the site of cell division in fission yeast. J Cell Biol. 1998;143:1603–1616. doi: 10.1083/jcb.143.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RW, Jeffrey PD, Zick M, Phillips BP, Wickner WT, Hughson FM. A direct role for the Sec1/Munc18-family protein Vps33 as a template for SNARE assembly. Science. 2015;349:1111–1114. doi: 10.1126/science.aac7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladrón V, Ufano S, Dueñas E, Martín-Cuadrado AB, del Rey F, Vázquez de Aldana CR. Eng1p, an endo-1,3-β-glucanase localized at the daughter side of the septum, is involved in cell separation in Saccharomyces cerevisiae. Eukaryot Cell. 2002;1:774–786. doi: 10.1128/ec.1.5.774-786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian MK, Bi E, Glotzer M. Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr Biol. 2004;14:R806–R818. doi: 10.1016/j.cub.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Baro B, Queralt E, Monje-Casas F. Regulation of mitotic exit in Saccharomyces cerevisiae. Methods Mol Biol. 2017;1505:3–17. doi: 10.1007/978-1-4939-6502-1_1. [DOI] [PubMed] [Google Scholar]
- Barr FA, Gruneberg U. Cytokinesis: placing and making the final cut. Cell. 2007;131:847–860. doi: 10.1016/j.cell.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Basant A, Glotzer M. Spatiotemporal regulation of RhoA during cytokinesis. Curr Biol. 2018;28:R570–R580. doi: 10.1016/j.cub.2018.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bement WM, Miller AL, von Dassow G. Rho GTPase activity zones and transient contractile arrays. Bioessays. 2006;28:983–993. doi: 10.1002/bies.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendezu FO, Martin SG (2011) Actin cables and the exocyst form two independent morphogenesis pathways in the fission yeast. Mol Biol Cell 22:44–53. 10.1091/mbc.E10-08-0720 [DOI] [PMC free article] [PubMed]
- Bendezu FO, Vincenzetti V, Martin SG. Fission yeast Sec3 and Exo70 are transported on actin cables and localize the exocyst complex to cell poles. PLoS One. 2012;7:e40248. doi: 10.1371/journal.pone.0040248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berro J, Pollard TD (2014) Local and global analysis of endocytic patch dynamics in fission yeast using a new “temporal superresolution” realignment method. Mol Biol Cell 25:3501–3514. 10.1091/mbc.E13-01-0004 [DOI] [PMC free article] [PubMed]
- Bertin A, et al. Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. PNAS. 2008;105:8274–8279. doi: 10.1073/pnas.0803330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestul AJ et al (2015) Fission yeast profilin is tailored to facilitate actin assembly by the cytokinesis formin Cdc12. Mol Biol Cell 26:283–293. 10.1091/mbc.E13-05-0281 [DOI] [PMC free article] [PubMed]
- Bhavsar-Jog YP, Bi E. Mechanics and regulation of cytokinesis in budding yeast. Semin Cell Dev Biol. 2017;66:107–118. doi: 10.1016/j.semcdb.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E, Maddox P, Lew DJ, Salmon ED, McMillan JN, Yeh E, Pringle JR. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J Cell Biol. 1998;142:1301–1312. doi: 10.1083/jcb.142.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidone TC, Tang H, Vavylonis D. Dynamic network morphology and tension buildup in a 3D model of cytokinetic ring assembly. Biophys J. 2014;107:2618–2628. doi: 10.1016/j.bpj.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluemink JG, de Laat SW. New membrane formation during cytokinesis in normal and cytochalasin B-treated eggs of Xenopus laevis. I. Electron microscope observations. J Cell Biol. 1973;59:89–108. doi: 10.1083/jcb.59.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucrot E, Pick A, Çamdere G, Liska N, Evergren E, McMahon Harvey T, Kozlov Michael M. Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent BAR domains. Cell. 2012;149:124–136. doi: 10.1016/j.cell.2012.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulant S, Kural C, Zeeh J-C, Ubelmann F, Kirchhausen T. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat Cell Biol. 2011;13:1124–1131. doi: 10.1038/ncb2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutté Y, et al. Endocytosis restricts Arabidopsis KNOLLE syntaxin to the cell division plane during late cytokinesis. EMBO J. 2010;29:546–558. doi: 10.1038/emboj.2009.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brach T, Godlee C, Moeller-Hansen I, Boeke D, Kaksonen M. The initiation of clathrin-mediated endocytosis is mechanistically highly flexible. Curr Biol. 2014;24:548–554. doi: 10.1016/j.cub.2014.01.048. [DOI] [PubMed] [Google Scholar]
- Brandhorst D, Zwilling D, Rizzoli SO, Lippert U, Lang T, Jahn R. Homotypic fusion of early endosomes: SNAREs do not determine fusion specificity. PNAS. 2006;103:2701–2706. doi: 10.1073/pnas.0511138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet S, Sacher M. Are all multisubunit tethering complexes bona fide tethers? Traffic. 2014;15:1282–1287. doi: 10.1111/tra.12200. [DOI] [PubMed] [Google Scholar]
- Byers B, Goetsch L. A highly ordered ring of membrane-associated filaments in budding yeast. J Cell Biol. 1976;69:717–721. doi: 10.1083/jcb.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E. The septation apparatus, a chitin-requiring machine in budding yeast. Arch Biochem Biophys. 2004;426:201–207. doi: 10.1016/j.abb.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Carroll SY, Stimpson HEM, Weinberg J, Toret CP, Sun Y, Drubin DG, Schmid SL. Analysis of yeast endocytic site formation and maturation through a regulatory transition point. Mol Biol Cell. 2012;23:657–668. doi: 10.1091/mbc.e11-02-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A, Desai A, Oegema K. Structural memory in the contractile ring makes the duration of cytokinesis independent of cell size. Cell. 2009;137:926–937. doi: 10.1016/j.cell.2009.03.021. [DOI] [PubMed] [Google Scholar]
- Chalamalasetty RB, Hümmer S, Nigg EA, Silljé HHW. Influence of human Ect2 depletion and overexpression on cleavage furrow formation and abscission. J Cell Sci. 2006;119:3008–3019. doi: 10.1242/jcs.03032. [DOI] [PubMed] [Google Scholar]
- Chant J. Cell polarity in yeast. Annu Rev Cell Dev Biol. 1999;15:365–391. doi: 10.1146/annurev.cellbio.15.1.365. [DOI] [PubMed] [Google Scholar]
- Chant J, Herskowitz I. Genetic control of bud site selection in yeast by a set of gene products that constitute a morphogenetic pathway. Cell. 1991;65:1203–1212. doi: 10.1016/0092-8674(91)90015-Q. [DOI] [PubMed] [Google Scholar]
- Chant J, Pringle JR. Patterns of bud-site selection in the yeast Saccharomyces cerevisiae. J Cell Biol. 1995;129:751–765. doi: 10.1083/jcb.129.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant J, Corrado K, Pringle JR, Herskowitz I. Yeast BUD5, encoding a putative GDP-GTP exchange factor, is necessary for bud site selection and interacts with bud formation gene BEM1. Cell. 1991;65:1213–1224. doi: 10.1016/0092-8674(91)90016-R. [DOI] [PubMed] [Google Scholar]
- Charras G, Paluch E. Blebs lead the way: how to migrate without lamellipodia. Nat Rev Mol Cell Biol. 2008;9:730–736. doi: 10.1038/nrm2453. [DOI] [PubMed] [Google Scholar]
- Cheffings TH, Burroughs Nigel J, Balasubramanian Mohan K. Actomyosin ring formation and tension generation in eukaryotic cytokinesis. Curr Biol. 2016;26:R719–R737. doi: 10.1016/j.cub.2016.06.071. [DOI] [PubMed] [Google Scholar]
- Chen W, Foss M, Tseng K-F, Zhang D. Redundant mechanisms recruit actin into the contractile ring in silkworm spermatocytes. PLoS Biol. 2008;6:e209. doi: 10.1371/journal.pbio.0060209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Arora PD, McCulloch CA, Wilde A. Cytokinesis requires localized β-actin filament production by an actin isoform specific nucleator. Nat Commun. 2017;8:1530. doi: 10.1038/s41467-017-01231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin CF, Tan K, Onishi M, Chew Y, Augustine B, Lee WR, Yeong FM. Timely endocytosis of cytokinetic enzymes prevents premature spindle breakage during mitotic exit. PLoS Genet. 2016;12:e1006195. doi: 10.1371/journal.pgen.1006195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JS, Schekman RW. Differential trafficking and timed localization of two chitin synthase proteins, Chs2p and Chs3p. J Cell Biol. 1996;135:597–610. doi: 10.1083/jcb.135.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman VC, Sees JA, Kovar DR, Wu J-Q. The formins Cdc12 and For3 cooperate during contractile ring assembly in cytokinesis. J Cell Biol. 2013;203:101–114. doi: 10.1083/jcb.201305022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés JCG, Ramos M, Osumi M, Pérez P, Ribas JC. Fission yeast septation. Commun Integr Biol. 2016;9:e1189045. doi: 10.1080/19420889.2016.1189045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucu B, Degreif D, Bertl A, Thiel G. Vesicle fusion and fission in plants and yeast. Cell Calcium. 2017;67:40–45. doi: 10.1016/j.ceca.2017.08.007. [DOI] [PubMed] [Google Scholar]
- Dambournet D, et al. Rab35 GTPase and OCRL phosphatase remodel lipids and F-actin for successful cytokinesis. Nat Cell Biol. 2011;13:981–988. doi: 10.1038/ncb2279. [DOI] [PubMed] [Google Scholar]
- Danilchik MV, Brown EE. Membrane dynamics of cleavage furrow closure in Xenopus laevis. Dev Dyn. 2008;237:565–579. doi: 10.1002/dvdy.21442. [DOI] [PubMed] [Google Scholar]
- Danilchik MV, Funk WC, Brown EE, Larkin K. Requirement for microtubules in new membrane formation during cytokinesis of Xenopus embryos. Dev Biol. 1998;194:47–60. doi: 10.1006/dbio.1997.8815. [DOI] [PubMed] [Google Scholar]
- Danilchik MV, Bedrick SD, Brown EE, Ray K. Furrow microtubules and localized exocytosis in cleaving Xenopus laevis embryos. J Cell Sci. 2003;116:273–283. doi: 10.1242/jcs.00217. [DOI] [PubMed] [Google Scholar]
- Daumke O, Roux A, Haucke V. BAR domain scaffolds in dynamin-mediated membrane fission. Cell. 2014;156:882–892. doi: 10.1016/j.cell.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Davidson R, Pontasch JA, Wu J-Q. Sbg1 is a novel regulator for the localization of the β-glucan synthase Bgs1 in fission yeast. PLoS One. 2016;11:e0167043. doi: 10.1371/journal.pone.0167043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Avino PP, Giansanti MG, Petronczki M. Cytokinesis in animal cells. Cold Spring Harb Perspect Biol. 2015;7:a015834. doi: 10.1101/cshperspect.a015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker N, Speijer D, Grun CH, van den Berg M, de Haan A, Hochstenbach F. Role of the α-glucanase Agn1p in fission-yeast cell separation. Mol Biol Cell. 2004;15:3903–3914. doi: 10.1091/mbc.e04-04-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Moseley JB (2013) Compartmentalized nodes control mitotic entry signaling in fission yeast. Mol Biol Cell 24:1872–1881. 10.1091/mbc.E13-02-0104 [DOI] [PMC free article] [PubMed]
- Dmitrieff S, Nédélec F. Membrane mechanics of endocytosis in cells with turgor. PLoS Comp Biol. 2015;11:e1004538. doi: 10.1371/journal.pcbi.1004538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere J, Barral Y. Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science. 2004;305:393–396. doi: 10.1126/science.1099892. [DOI] [PubMed] [Google Scholar]
- Douglas LM, Alvarez FJ, McCreary C, Konopka JB. Septin function in yeast model systems and pathogenic fungi. Eukaryot Cell. 2005;4:1503–1512. doi: 10.1128/ec.4.9.1503-1512.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuke ML, Munson M. The secret life of tethers: the role of tethering factors in SNARE complex regulation. Front Cell Dev Biol. 2016;4:42. doi: 10.3389/fcell.2016.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faty M, Fink M, Barral Y. Septins: a ring to part mother and daughter. Curr Genet. 2002;41:123–131. doi: 10.1007/s00294-002-0304-0. [DOI] [PubMed] [Google Scholar]
- Feng B, Schwarz H, Jesuthasan S (2002) Furrow-specific endocytosis during cytokinesis of Zebrafish blastomeres. Exp Cell Res 279:14–20. 10.1006/excr.2002.5579 [DOI] [PubMed]
- Fielding AB, et al. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 2005;24:3389–3399. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltman M, Molist I, Arcones I, Sacristan C, Filali-Mouncef Y, Roncero C, Sanchez-Diaz A. Ingression progression complexes control extracellular matrix remodelling during cytokinesis in budding yeast. PLoS Genet. 2016;12:e1005864. doi: 10.1371/journal.pgen.1005864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltman M, Filali-Mouncef Y, Crespo D, Sanchez-Diaz A. Cell polarity protein Spa2 coordinates Chs2 incorporation at the division site in budding yeast. PLoS Genet. 2018;14:e1007299. doi: 10.1371/journal.pgen.1007299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremont S, Echard A. Membrane traffic in the late steps of cytokinesis. Curr Biol. 2018;28:R458–R470. doi: 10.1016/j.cub.2018.01.019. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–1047. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev. 2007;17:157–162. doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Garcia G, 3rd, Bertin A, Li Z, Song Y, McMurray MA, Thorner J, Nogales E. Subunit-dependent modulation of septin assembly: budding yeast septin Shs1 promotes ring and gauze formation. J Cell Biol. 2011;195:993–1004. doi: 10.1083/jcb.201107123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerald NJ, Damer CK, O'Halloran TJ, De Lozanne A. Cytokinesis failure in clathrin-minus cells is caused by cleavage furrow instability. Cell Motility. 2001;48:213–223. doi: 10.1002/1097-0169(200103)48:3<213::AID-CM1010>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Gladfelter AS, Pringle JR, Lew DJ. The septin cortex at the yeast mother–bud neck. Curr Opin Microbiol. 2001;4:681–689. doi: 10.1016/S1369-5274(01)00269-7. [DOI] [PubMed] [Google Scholar]
- Glomb O, Gronemeyer T. Septin organization and functions in budding yeast. Front Cell Dev Biol. 2016;4:123. doi: 10.3389/fcell.2016.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- Goode BL, Eskin JA, Wendland B. Actin and endocytosis in budding yeast. Genetics. 2015;199:315–358. doi: 10.1534/genetics.112.145540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss JW, Toomre DK. Both daughter cells traffic and exocytose membrane at the cleavage furrow during mammalian cytokinesis. J Cell Biol. 2008;181:1047–1054. doi: 10.1083/jcb.200712137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal A, Takaine M, Simanis V, Nakano K. Dividing the spoils of growth and the cell cycle: the fission yeast as a model for the study of cytokinesis. Cytoskeleton. 2011;68:69–88. doi: 10.1002/cm.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan R, Dai H, Rizo J. Binding of the Munc13-1 MUN domain to membrane-anchored SNARE complexes. Biochemistry. 2008;47:1474–1481. doi: 10.1021/bi702345m. [DOI] [PubMed] [Google Scholar]
- Guo W, Tamanoi F, Novick P. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat Cell Biol. 2001;3:353–360. doi: 10.1038/35070029. [DOI] [PubMed] [Google Scholar]
- Gupta DK, Du J, Kamranvar SA, Johansson S. Tension-induced cytokinetic abscission in human fibroblasts. Oncotarget. 2018;9:8999–9009. doi: 10.18632/oncotarget.24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Vendrell M, Rincon SA, Dingli F, Loew D, Paoletti A. Molecular control of the Wee1 regulatory pathway by the SAD kinase Cdr2. J Cell Sci. 2015;128:2842–2853. doi: 10.1242/jcs.173146. [DOI] [PubMed] [Google Scholar]
- Harkins HA, et al. Bud8p and Bud9p, proteins that may mark the sites for bipolar budding in yeast. Mol Biol Cell. 2001;12:2497–2518. doi: 10.1091/mbc.12.8.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne WM, Boucrot E, Meinecke M, Evergren E, Vallis Y, Mittal R, McMahon HT. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science. 2010;328:1281–1284. doi: 10.1126/science.1188462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson GR, O'Farrell PH. Rho-dependent control of anillin behavior during cytokinesis. J Cell Biol. 2008;180:285–294. doi: 10.1083/jcb.200709005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa T et al (2010) Impairment of embryonic cell division and glycosaminoglycan biosynthesis in glucuronyltransferase-I-deficient mice. J Biol Chem 285:12190–12196. 10.1074/jbc.M110.100941 [DOI] [PMC free article] [PubMed]
- Izumikawa T, Sato B, Kitagawa H. Chondroitin sulfate is indispensable for pluripotency and differentiation of mouse embryonic stem cells. Sci Rep. 2014;4:3701. doi: 10.1038/srep03701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber E, et al. A putative TRAPPII tethering factor is required for cell plate assembly during cytokinesis in Arabidopsis. New Phytol. 2010;187:751–763. doi: 10.1111/j.1469-8137.2010.03331.x. [DOI] [PubMed] [Google Scholar]
- Jäntti J, Aalto MK, Oyen M, Sundqvist L, Keränen S, Ronne H. Characterization of temperature-sensitive mutations in the yeast syntaxin 1 homologues Sso1p and Sso2p, and evidence of a distinct function for Sso1p in sporulation. J Cell Sci. 2002;115:409–420. doi: 10.1242/jcs.115.2.409. [DOI] [PubMed] [Google Scholar]
- Jesuthasan S. Furrow-associated microtubule arrays are required for the cohesion of zebrafish blastomeres following cytokinesis. J Cell Sci. 1998;111:3695–3703. doi: 10.1242/jcs.111.24.3695. [DOI] [PubMed] [Google Scholar]
- Jin Y, et al. Myosin V transports secretory vesicles via a Rab GTPase cascade and interaction with the exocyst complex. Dev Cell. 2011;21:1156–1170. doi: 10.1016/j.devcel.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain I, Dooley HC, Toda T. Fission yeast sec3 bridges the exocyst complex to the actin cytoskeleton. Traffic. 2012;13:1481–1495. doi: 10.1111/j.1600-0854.2012.01408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens G, Park M, Richter S, Touihri S, Krause C, El Kasmi F, Mayer U. Plant cytokinesis: a tale of membrane traffic and fusion. Biochem Soc Trans. 2015;43:73–78. doi: 10.1042/bst20140246. [DOI] [PubMed] [Google Scholar]
- Kaksonen M, Roux A. Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2018;19:313–326. doi: 10.1038/nrm.2017.132. [DOI] [PubMed] [Google Scholar]
- Kang PJ, Lee ME, Park H-O. Bud3 activates Cdc42 to establish a proper growth site in budding yeast. J Cell Biol. 2014;206:19–28. doi: 10.1083/jcb.201402040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang PJ, Miller KE, Guegueniat J, Beven L, Park H-O (2018) The shared role of the Rsr1 GTPase and Gic1/Gic2 in Cdc42 polarization. Mol Biol Cell:mbcE18020145. 10.1091/mbc.E18-02-0145 [DOI] [PMC free article] [PubMed]
- Karnahl M, Park M, Krause C, Hiller U, Mayer U, Stierhof Y-D, Jürgens G. Functional diversification of Arabidopsis SEC1-related SM proteins in cytokinetic and secretory membrane fusion. PNAS. 2018;115:6309–6314. doi: 10.1073/pnas.1722611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Kawasaki H, Ohyama Y, Morishita T, Iwasaki H, Kokubo T, Hirano H. Cell polarity in Saccharomyces cerevisiae depends on proper localization of the Bud9 landmark protein by the EKC/KEOPS complex. Genetics. 2011;188:871–882. doi: 10.1534/genetics.111.128231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BT, Graham SC, Liska N, Dannhauser PN, Höning S, Ungewickell EJ, Owen DJ. AP2 controls clathrin polymerization with a membrane-activated switch. Science. 2014;345:459–463. doi: 10.1126/science.1254836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliullin RN, Green RA, Shi LZ, Gomez-Cavazos JS, Berns MW, Desai A, Oegema K. A positive-feedback-based mechanism for constriction rate acceleration during cytokinesis in. eLife. 2018;7:e36073. doi: 10.7554/eLife.36073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkert K, Echard A. Rab35 GTPase: a central regulator of phosphoinositides and F-actin in endocytic recycling and beyond. Traffic. 2016;17:1063–1077. doi: 10.1111/tra.12422. [DOI] [PubMed] [Google Scholar]
- Kukulski W, Schorb M, Kaksonen M, Briggs JA. Plasma membrane reshaping during endocytosis is revealed by time-resolved electron tomography. Cell. 2012;150:508–520. doi: 10.1016/j.cell.2012.05.046. [DOI] [PubMed] [Google Scholar]
- Kukulski W, Schorb M, Kaksonen M, Briggs John AG. Plasma membrane reshaping during endocytosis is revealed by time-resolved electron tomography. Cell. 2012;150:508–520. doi: 10.1016/j.cell.2012.05.046. [DOI] [PubMed] [Google Scholar]
- Lacroix B, Maddox AS. Cytokinesis, ploidy and aneuploidy. J Pathol. 2012;226:338–351. doi: 10.1002/path.3013. [DOI] [PubMed] [Google Scholar]
- Laplante C, Huang F, Tebbs IR, Bewersdorf J, Pollard TD. Molecular organization of cytokinesis nodes and contractile rings by super-resolution fluorescence microscopy of live fission yeast. PNAS. 2016;113:E5876–E5885. doi: 10.1073/pnas.1608252113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte D, Coffman VC, Lee I-J, Wu J-Q. Assembly and architecture of precursor nodes during fission yeast cytokinesis. J Cell Biol. 2011;192:1005–1021. doi: 10.1083/jcb.201008171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber MH, et al. The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J Cell Biol. 1997;139:1485–1493. doi: 10.1083/jcb.139.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I-J, Wu J-Q. Characterization of Mid1 domains for targeting and scaffolding in fission yeast cytokinesis. J Cell Sci. 2012;125:2973–2985. doi: 10.1242/jcs.102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I-J, Coffman VC, Wu J-Q. Contractile-ring assembly in fission yeast cytokinesis: recent advances and new perspectives. Cytoskeleton. 2012;69:751–763. doi: 10.1002/cm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ME, Lo W-C, Miller KE, Chou C-S, Park H-O. Regulation of Cdc42 polarization by the Rsr1 GTPase and Rga1, a Cdc42 GTPase-activating protein, in budding yeast. J Cell Sci. 2015;128:2106–2117. doi: 10.1242/jcs.166538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz M, Thoresen T, Gardel ML, Dinner AR. Contractile units in disordered actomyosin bundles arise from F-actin buckling. Phys Rev Lett. 2012;108:238107. doi: 10.1103/PhysRevLett.108.238107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore DM, Martínez-Núñez L, Munson M. Exposing the elusive exocyst structure. Trends Biochem Sci. 2018;43:714–725. doi: 10.1016/j.tibs.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewellyn L, Carvalho A, Desai A, Maddox AS, Oegema K. The chromosomal passenger complex and centralspindlin independently contribute to contractile ring assembly. J Cell Biol. 2011;193:155–169. doi: 10.1083/jcb.201008138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka E, et al. The phragmoplast-orienting Kinesin-12 class proteins translate the positional information of the preprophase band to establish the cortical division zone in Arabidopsis thaliana. Plant Cell. 2014;26:2617–2632. doi: 10.1105/tpc.114.124933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott J, Li R. Sequential assembly of Myosin II, an IQGAP-like protein, and filamentous actin to a ring structure involved in budding yeast cytokinesis. J Cell Biol. 1998;140:355–366. doi: 10.1083/jcb.140.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott J, Li R. Involvement of PCH family proteins in cytokinesis and actin distribution. Microsc Res Tech. 2000;49:168–172. doi: 10.1002/(SICI)1097-0029(20000415)49:2<168::AID-JEMT9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Lippincott J, Shannon KB, Shou W, Deshaies RJ, Li R. The Tem1 small GTPase controls actomyosin and septin dynamics during cytokinesis. J Cell Sci. 2001;114:1379–1386. doi: 10.1242/jcs.114.7.1379. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wu J-Q. Cytokinesis: going super-resolution in live cells. Curr Biol. 2016;26:R1150–R1152. doi: 10.1016/j.cub.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q et al (2016a) MICAL3 flavoprotein monooxygenase forms a complex with centralspindlin and regulates cytokinesis. J Biol Chem 291:20617–20629. 10.1074/jbc.M116.748186 [DOI] [PMC free article] [PubMed]
- Liu Y, Lee I-J, Sun M, Lower CA, Runge KW, Ma J, Wu J-Q (2016b) Roles of the novel coiled-coil protein Rng10 in septum formation during fission yeast cytokinesis. Mol Biol Cell 27:2528–2541. 10.1091/mbc.E16-03-0156 [DOI] [PMC free article] [PubMed]
- Longtine MS, DeMarini DJ, Valencik ML, Al-Awar OS, Fares H, De Virgilio C, Pringle JR. The septins: roles in cytokinesis and other processes. Curr Opin Cell Biol. 1996;8:106–119. doi: 10.1016/S0955-0674(96)80054-8. [DOI] [PubMed] [Google Scholar]
- Lord M, Laves E, Pollard TD. Cytokinesis depends on the motor domains of myosin-II in fission yeast but not in budding yeast. Mol Biol Cell. 2005;16:5346–5355. doi: 10.1091/mbc.e05-07-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Zhang J, Guo W (2014) The role of Sec3p in secretory vesicle targeting and exocyst complex assembly. Mol Biol Cell 25:3813–3822. 10.1091/mbc.E14-04-0907 [DOI] [PMC free article] [PubMed]
- Ma C, Li W, Xu Y, Rizo J. Munc13 mediates the transition from the closed syntaxin–Munc18 complex to the SNARE complex. Nat Struct Mol Biol. 2011;18:542–549. doi: 10.1038/nsmb.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SG, Berthelot-Grosjean M. Polar gradients of the DYRK-family kinase Pom1 couple cell length with the cell cycle. Nature. 2009;459:852–856. doi: 10.1038/nature08054. [DOI] [PubMed] [Google Scholar]
- Martín-Cuadrado AB et al (2005) Role of septins and the exocyst complex in the function of hydrolytic enzymes responsible for fission yeast cell separation. Mol Biol Cell 16:4867–4881. 10.1091/mbc.E04-12-1114 [DOI] [PMC free article] [PubMed]
- Martinez P, Luo A, Sylvester A, Rasmussen CG. Proper division plane orientation and mitotic progression together allow normal growth of maize. PNAS. 2017;114:2759–2764. doi: 10.1073/pnas.1619252114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupin P, Phillips CL, Adelstein RS, Pollard TD. Differential localization of myosin-II isozymes in human cultured cells and blood cells. J Cell Sci. 1994;107:3077–3090. doi: 10.1242/jcs.107.11.3077. [DOI] [PubMed] [Google Scholar]
- Mayor S, Parton RG, Donaldson JG. Clathrin-independent pathways of endocytosis. Cold Spring Harb Perspect Biol. 2014;6:a016758. doi: 10.1101/cshperspect.a016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum D, Gould KL. Timing is everything: regulation of mitotic exit and cytokinesis by the MEN and SIN. Trends Cell Biol. 2001;11:89–95. doi: 10.1016/S0962-8924(00)01901-2. [DOI] [PubMed] [Google Scholar]
- McCusker D, Kellogg DR. Plasma membrane growth during the cell cycle: unsolved mysteries and recent progress. Curr Opin Cell Biol. 2012;24:845–851. doi: 10.1016/j.ceb.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald NA, Lind AL, Smith SE, Li R, Gould KL. Nanoscale architecture of the Schizosaccharomyces pombe contractile ring. eLife. 2017;6:e28865. doi: 10.7554/eLife.28865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei K, et al. Cryo-EM structure of the exocyst complex. Nat Struct Mol Biol. 2018;25:139–146. doi: 10.1038/s41594-017-0016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitinger F, Palani S. Actomyosin ring driven cytokinesis in budding yeast. Semin Cell Dev Biol. 2016;53:19–27. doi: 10.1016/j.semcdb.2016.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes Pinto I, Rubinstein B, Kucharavy A, Unruh Jay R, Li R. Actin depolymerization drives actomyosin ring contraction during budding yeast cytokinesis. Dev Cell. 2012;22:1247–1260. doi: 10.1016/j.devcel.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Lo WC, Lee ME, Kang PJ, Park H-O (2017) Fine-tuning the orientation of the polarity axis by Rga1, a Cdc42 GTPase-activating protein. Mol Biol Cell 28:3773–3788. 10.1091/mbc.E17-01-0074 [DOI] [PMC free article] [PubMed]
- Mir R, Morris VH, Buschmann H, Rasmussen CG. Division plane orientation defects revealed by a synthetic double mutant phenotype. Plant Physiol. 2018;176:418–431. doi: 10.1104/pp.17.01075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi S, et al. Chondroitin proteoglycans are involved in cell division of Caenorhabditis elegans. Nature. 2003;423:443–448. doi: 10.1038/nature01635. [DOI] [PubMed] [Google Scholar]
- Moseley JB, Nurse P. Cdk1 and cell morphology: connections and directions. Curr Opin Cell Biol. 2009;21:82–88. doi: 10.1016/j.ceb.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Moseley JB, Nurse P. Cell division intersects with cell geometry. Cell. 2010;142:189–193. doi: 10.1016/j.cell.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Moseley JB, Mayeux A, Paoletti A, Nurse P. A spatial gradient coordinates cell size and mitotic entry in fission yeast. Nature. 2009;459:857–860. doi: 10.1038/nature08074. [DOI] [PubMed] [Google Scholar]
- Muñoz J, et al. Extracellular cell wall β(1,3) glucan is required to couple septation to actomyosin ring contraction. J Cell Biol. 2013;203:265–282. doi: 10.1083/jcb.201304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, et al. Mechanism of microtubule array expansion in the cytokinetic phragmoplast. Nat Commun. 2013;4:1967. doi: 10.1038/ncomms2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto H, Kaupisch A, Collins LL, Gould GW (2013) Syntaxin 16 is a master recruitment factor for cytokinesis. Mol Biol Cell 24:3663–3674. 10.1091/mbc.E13-06-0302 [DOI] [PMC free article] [PubMed]
- Newmyer SL, Christensen A, Sever S. Auxilin-dynamin interactions link the uncoating ATPase chaperone machinery with vesicle formation. Dev Cell. 2003;4:929–940. doi: 10.1016/S1534-5807(03)00157-6. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Yonemura S. Centralspindlin regulates ECT2 and RhoA accumulation at the equatorial cortex during cytokinesis. J Cell Sci. 2006;119:104–114. doi: 10.1242/jcs.02737. [DOI] [PubMed] [Google Scholar]
- Noordstra I, Akhmanova A. Linking cortical microtubule attachment and exocytosis. F1000Res. 2017;6:469. doi: 10.12688/f1000research.10729.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelz DB, Rubinstein Boris Y, Mogilner A. A combination of actin treadmilling and cross-linking drives contraction of random actomyosin arrays. Biophys J. 2015;109:1818–1829. doi: 10.1016/j.bpj.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y, et al. Hof1 and Chs4 interact via F-BAR domain and Sel1-like repeats to control extracellular matrix deposition during cytokinesis. Curr Biol. 2017;27:2878-2886.e2875. doi: 10.1016/j.cub.2017.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojkic N, Wu J-Q, Vavylonis D. Model of myosin node aggregation into a contractile ring: the effect of local alignment. J Phys Condens Matter. 2011;23:374103. doi: 10.1088/0953-8984/23/37/374103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Leda M, Hanna J, Savage Natasha S, Bi E, Goryachev Andrew B. Daughter cell identity emerges from the interplay of Cdc42, septins, and exocytosis. Dev Cell. 2013;26:148–161. doi: 10.1016/j.devcel.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SK, Bishop JR, Yates JR, Oegema K, Esko JD. Identification of novel chondroitin proteoglycans in Caenorhabditis elegans: embryonic cell division depends on CPG-1 and CPG-2. J Cell Biol. 2006;173:985–994. doi: 10.1083/jcb.200603003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong K, Wloka C, Okada S, Svitkina T, Bi E. Architecture and dynamic remodelling of the septin cytoskeleton during the cell cycle. Nat Commun. 2014;5:5698. doi: 10.1038/ncomms6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, et al. IQGAP-related Rng2p organizes cortical nodes and ensures position of cell division in fission yeast. Curr Biol. 2011;21:467–472. doi: 10.1016/j.cub.2011.01.059. [DOI] [PubMed] [Google Scholar]
- Paoletti A, Chang F, Stearns T. Analysis of mid1p, a protein required for placement of the cell division site, reveals a link between the nucleus and the cell surface in fission yeast. Mol Biol Cell. 2000;11:2757–2773. doi: 10.1091/mbc.11.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolo D'Avino P. How to scaffold the contractile ring for a safe cytokinesis—lessons from anillin-related proteins. J Cell Sci. 2009;122:1071–1079. doi: 10.1242/jcs.034785. [DOI] [PubMed] [Google Scholar]
- Papadopulos A. Membrane shaping by actin and myosin during regulated exocytosis. Mol Cell Neurosci. 2017;84:93–99. doi: 10.1016/j.mcn.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Pérez P, Portales E, Santos B. Rho4 interaction with exocyst and septins regulates cell separation in fission yeast. Microbiology. 2015;161:948–959. doi: 10.1099/mic.0.000062. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR (2017) Rab GTPases: master regulators that establish the secretory and endocytic pathways. Mol Biol Cell 28:712–715. 10.1091/mbc.E16-10-0737 [DOI] [PMC free article] [PubMed]
- Picco A, et al. The in vivo architecture of the exocyst provides structural basis for exocytosis. Cell. 2017;168:400–412. doi: 10.1016/j.cell.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Pollard TD. Nine unanswered questions about cytokinesis. J Cell Biol. 2017;216:3007–3016. doi: 10.1083/jcb.201612068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, Wu J-Q. Understanding cytokinesis: lessons from fission yeast. Nat Rev Mol Cell Biol. 2010;11:149–155. doi: 10.1038/nrm2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posor Y, Eichhorn-Grünig M, Haucke V. Phosphoinositides in endocytosis. Biochim Biophys Acta. 2015;1851:794–804. doi: 10.1016/j.bbalip.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Gould GW. Breaking up is hard to do—membrane traffic in cytokinesis. J Cell Sci. 2008;121:1569–1576. doi: 10.1242/jcs.018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor SA, Minc N, Boudaoud A, Chang F. Contributions of turgor pressure, the contractile ring, and septum assembly to forces in cytokinesis in fission yeast. Curr Biol. 2012;22:1601–1608. doi: 10.1016/j.cub.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadota H, et al. Identification of yeast Rho1 p GTPase as a regulatory subunit of 1,3-β-glucan synthase. Science. 1996;272:279–281. doi: 10.1126/science.272.5259.279. [DOI] [PubMed] [Google Scholar]
- Qi X, Kaneda M, Chen J, Geitmann A, Zheng H. A specific role for Arabidopsis TRAPPII in post-Golgi trafficking that is crucial for cytokinesis and cell polarity. Plant J. 2011;68:234–248. doi: 10.1111/j.1365-313X.2011.04681.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen CG, Bellinger M. An overview of plant division-plane orientation. New Phytol. 2018;219:505–512. doi: 10.1111/nph.15183. [DOI] [PubMed] [Google Scholar]
- Rasmussen CG, Sun B, Smith LG. Tangled localization at the cortical division site of plant cells occurs by several mechanisms. J Cell Sci. 2011;124:270–279. doi: 10.1242/jcs.073676. [DOI] [PubMed] [Google Scholar]
- Rasmussen CG, Wright AJ, Muller S. The role of the cytoskeleton and associated proteins in determination of the plant cell division plane. Plant J. 2013;75:258–269. doi: 10.1111/tpj.12177. [DOI] [PubMed] [Google Scholar]
- Rincon SA, Paoletti A. Mid1/anillin and the spatial regulation of cytokinesis in fission yeast. Cytoskeleton. 2012;69:764–777. doi: 10.1002/cm.21056. [DOI] [PubMed] [Google Scholar]
- Rincon SA, Paoletti A. Molecular control of fission yeast cytokinesis. Semin Cell Dev Biol. 2016;53:28–38. doi: 10.1016/j.semcdb.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Rincon SA, et al. Pom1 regulates the assembly of Cdr2–Mid1 cortical nodes for robust spatial control of cytokinesis. J Cell Biol. 2014;206:61–77. doi: 10.1083/jcb.201311097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinett CC, Giansanti MG, Gatti M, Fuller MT. TRAPPII is required for cleavage furrow ingression and localization of Rab11 in dividing male meiotic cells of Drosophila. J Cell Sci. 2009;122:4526–4534. doi: 10.1242/jcs.054536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer T, Madden K, Chang J, Snyder M. Selection of axial growth sites in yeast requires Axl2p, a novel plasma membrane glycoprotein. Genes Dev. 1996;10:777–793. doi: 10.1101/gad.10.7.777. [DOI] [PubMed] [Google Scholar]
- Rossi G, Watson K, Kennedy W, Brennwald P (2018) The tomosyn homologue, Sro7, is a direct effector of the Rab GTPase, Sec4, in post-Golgi vesicle tethering. Mol Biol Cell 29:1476–1486. 10.1091/mbc.E18-02-0138 [DOI] [PMC free article] [PubMed]
- Rybak K, et al. Plant cytokinesis is orchestrated by the sequential action of the TRAPPII and exocyst tethering complexes. Dev Cell. 2014;29:607–620. doi: 10.1016/j.devcel.2014.04.029. [DOI] [PubMed] [Google Scholar]
- Ryu J-K, et al. Spring-loaded unraveling of a single SNARE complex by NSF in one round of ATP turnover. Science. 2015;347:1485–1489. doi: 10.1126/science.aaa5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagona AP, Stenmark H. Cytokinesis and cancer. FEBS Lett. 2010;584:2652–2661. doi: 10.1016/j.febslet.2010.03.044. [DOI] [PubMed] [Google Scholar]
- Salbreux G, Prost J, Joanny JF. Hydrodynamics of cellular cortical flows and the formation of contractile rings. Phys Rev Lett. 2009;103:058102. doi: 10.1103/PhysRevLett.103.058102. [DOI] [PubMed] [Google Scholar]
- Sburlati A, Cabib E. Chitin synthetase 2, a presumptive participant in septum formation in Saccharomyces cerevisiae. J Biol Chem. 1986;261:15147–15152. [PubMed] [Google Scholar]
- Schenkman LR, Caruso C, Page N, Pringle JR. The role of cell cycle-regulated expression in the localization of spatial landmark proteins in yeast. J Cell Biol. 2002;156:829–841. doi: 10.1083/jcb.200107041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiel JA, Simon GC, Zaharris C, Weisz J, Castle D, Wu CC, Prekeris R. FIP3-endosome-dependent formation of the secondary ingression mediates ESCRT-III recruitment during cytokinesis. Nat Cell Biol. 2012;14:1068–1078. doi: 10.1038/ncb2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedzinski J, Biro M, Oswald A, Tinevez J-Y, Salbreux G, Paluch E. Polar actomyosin contractility destabilizes the position of the cytokinetic furrow. Nature. 2011;476:462–466. doi: 10.1038/nature10286. [DOI] [PubMed] [Google Scholar]
- Segui-Simarro JM, Staehelin LA. Cell cycle-dependent changes in Golgi stacks, vacuoles, clathrin-coated vesicles and multivesicular bodies in meristematic cells of Arabidopsis thaliana: a quantitative and spatial analysis. Planta. 2006;223:223–236. doi: 10.1007/s00425-005-0082-2. [DOI] [PubMed] [Google Scholar]
- Shuster CB, Burgess DR. Transitions regulating the timing of cytokinesis in embryonic cells. Curr Biol. 2002;12:854–858. doi: 10.1016/S0960-9822(02)00838-2. [DOI] [PubMed] [Google Scholar]
- Simanis V. Pombe’s thirteen—control of fission yeast cell division by the septation initiation network. J Cell Sci. 2015;128:1465–1474. doi: 10.1242/jcs.094821. [DOI] [PubMed] [Google Scholar]
- Skop AR, Liu H, Yates J, 3rd, Meyer BJ, Heald R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 2004;305:61–66. doi: 10.1126/science.1097931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smertenko A (2018) Phragmoplast expansion: the four-stroke engine that powers plant cytokinesis. Curr Opin Plant Biol. 10.1016/j.pbi.2018.07.011 [DOI] [PubMed]
- Smith LG, Hake S, Sylvester AW. The tangled-1 mutation alters cell division orientations throughout maize leaf development without altering leaf shape. Development. 1996;122:481–489. doi: 10.1242/dev.122.2.481. [DOI] [PubMed] [Google Scholar]
- Sousa R, Lafer EM. The role of molecular chaperones in clathrin mediated vesicular trafficking. Front Mol Biosci. 2015;2:26. doi: 10.3389/fmolb.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinner L, et al. A protein phosphatase 2A complex spatially controls plant cell division. Nat Commun. 2013;4:1863. doi: 10.1038/ncomms2831. [DOI] [PubMed] [Google Scholar]
- Stachowiak MR, Laplante C, Chin Harvey F, Guirao B, Karatekin E, Pollard TD, O’Shaughnessy B. Mechanism of cytokinetic contractile ring constriction in fission yeast. Dev Cell. 2014;29:547–561. doi: 10.1016/j.devcel.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin LA, Hepler PK. Cytokinesis in higher plants. Cell. 1996;84:821–824. doi: 10.1016/S0092-8674(00)81060-0. [DOI] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nat Rev Mol Cell Biol. 2004;5:45–54. doi: 10.1038/nrm1276. [DOI] [PubMed] [Google Scholar]
- Sun L, et al. Mechanistic insights into the anchorage of the contractile ring by anillin and Mid1. Dev Cell. 2015;33:413–426. doi: 10.1016/j.devcel.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Leong NT, Wong T, Drubin DG, Pollard TD. A Pan1/End3/Sla1 complex links Arp2/3-mediated actin assembly to sites of clathrin-mediated endocytosis. Mol Biol Cell. 2015;26:3841–3856. doi: 10.1091/mbc.e15-04-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara H, et al. Clathrin is involved in organization of mitotic spindle and phragmoplast as well as in endocytosis in tobacco cell cultures. Protoplasma. 2007;230:1–11. doi: 10.1007/s00709-006-0226-7. [DOI] [PubMed] [Google Scholar]
- Takizawa PA, DeRisi JL, Wilhelm JE, Vale RD. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science. 2000;290:341–344. doi: 10.1126/science.290.5490.341. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Ito A, Saito M. A model of muscle contraction based on the Langevin equation with actomyosin potentials. Comput Methods Biomech Biomed Engin. 2017;20:273–283. doi: 10.1080/10255842.2016.1215440. [DOI] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P. The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. doi: 10.1002/j.1460-2075.1996.tb01039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiyagarajan S, Munteanu EL, Arasada R, Pollard TD, O’Shaughnessy B. The fission yeast cytokinetic contractile ring regulates septum shape and closure. J Cell Sci. 2015;128:3672–3681. doi: 10.1242/jcs.166926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z, Gao X-D, Howell AS, Bose I, Lew DJ, Bi E. Adjacent positioning of cellular structures enabled by a Cdc42 GTPase-activating protein-mediated zone of inhibition. J Cell Biol. 2007;179:1375–1384. doi: 10.1083/jcb.200705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PT, Marsh L, Doye V, Inoué S, Chang F. A mechanism for nuclear positioning in fission yeast based on microtubule pushing. J Cell Biol. 2001;153:397–412. doi: 10.1083/jcb.153.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E. The 70-kd mammalian heat shock proteins are structurally and functionally related to the uncoating protein that releases clathrin triskelia from coated vesicles. EMBO J. 1985;4:3385–3391. doi: 10.1002/j.1460-2075.1985.tb04094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia RH, Schekman R. The yeasts Rho1p and Pkc1p regulate the transport of chitin synthase III (Chs3p) from internal stores to the plasma membrane. PNAS. 2003;100:10287–10292. doi: 10.1073/pnas.1834246100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme D, et al. Adaptin-like protein TPLATE and clathrin recruitment during plant somatic cytokinesis occurs via two distinct pathways. PNAS. 2011;108:615–620. doi: 10.1073/pnas.1017890108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oostende-Triplet C, Guillet D, Triplet T, Pandzic E, Wiseman PW, Geitmann A. Vesicle dynamics during plant cell cytokinesis reveals distinct developmental phases. Plant Physiol. 2017;174:1544–1558. doi: 10.1104/pp.17.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavylonis D, Wu J-Q, Hao S, O'Shaughnessy B, Pollard TD. Assembly mechanism of the contractile ring for cytokinesis by fission yeast. Science. 2008;319:97–100. doi: 10.1126/science.1151086. [DOI] [PubMed] [Google Scholar]
- VerPlank L, Li R. Cell cycle-regulated trafficking of Chs2 controls actomyosin ring stability during cytokinesis. Mol Biol Cell. 2005;16:2529–2543. doi: 10.1091/mbc.e04-12-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrabioiu AM, Mitchison TJ. Structural insights into yeast septin organization from polarized fluorescence microscopy. Nature. 2006;443:466–469. doi: 10.1038/nature05109. [DOI] [PubMed] [Google Scholar]
- Wagner E, Glotzer M. Local RhoA activation induces cytokinetic furrows independent of spindle position and cell cycle stage. J Cell Biol. 2016;213:641–649. doi: 10.1083/jcb.201603025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KL, Muller S, Moss D, Ehrhardt DW, Smith LG. Arabidopsis TANGLED identifies the division plane throughout mitosis and cytokinesis. Curr Biol. 2007;17:1827–1836. doi: 10.1016/j.cub.2007.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tang X, Liu J, Trautmann S, Balasundaram D, McCollum D, Balasubramanian MK. The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol Biol Cell. 2002;13:515–529. doi: 10.1091/mbc.01-11-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Wang M, Zhu Y-H, Grosel TW, Sun D, Kudryashov DS, Wu J-Q (2015) The Rho-GEF Gef3 interacts with the septin complex and activates the GTPase Rho4 during fission yeast cytokinesis. Mol Biol Cell 26:238–255. 10.1091/mbc.E14-07-1196 [DOI] [PMC free article] [PubMed]
- Wang N, Lee I-J, Rask G, Wu J-Q. Roles of the TRAPP-II complex and the exocyst in membrane deposition during fission yeast cytokinesis. PLoS Biol. 2016;14:e1002437. doi: 10.1371/journal.pbio.1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Bednarek S. Cytokinesis: GAGs form the walls that separate our parts. Curr Biol. 2003;13:R717–R718. doi: 10.1016/j.cub.2003.08.048. [DOI] [PubMed] [Google Scholar]
- Whyte JRC, Munro S. Vesicle tethering complexes in membrane traffic. J Cell Sci. 2002;115:2627–2637. doi: 10.1242/jcs.115.13.2627. [DOI] [PubMed] [Google Scholar]
- Wickner W, Rizo J (2017) A cascade of multiple proteins and lipids catalyzes membrane fusion. Mol Biol Cell 28:707–711. 10.1091/mbc.E16-07-0517 [DOI] [PMC free article] [PubMed]
- Wideman JG, Leung KF, Field MC, Dacks JB. The cell biology of the endocytic system from an evolutionary perspective. Cold Spring Harb Perspect Biol. 2014;6:a016998. doi: 10.1101/cshperspect.a016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk S, Wittland J, Thywissen A, Schmitz HP, Heinisch JJ. A block of endocytosis of the yeast cell wall integrity sensors Wsc1 and Wsc2 results in reduced fitness in vivo. Mol Gen Genomics. 2010;284:217–229. doi: 10.1007/s00438-010-0563-2. [DOI] [PubMed] [Google Scholar]
- Willet AH, McDonald NA, Gould KL. Regulation of contractile ring formation and septation in Schizosaccharomyces pombe. Curr Opin Microbiol. 2015;28:46–52. doi: 10.1016/j.mib.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloka C, Bi E. Mechanisms of cytokinesis in budding yeast. Cytoskeleton. 2012;69:710–726. doi: 10.1002/cm.21046. [DOI] [PubMed] [Google Scholar]
- Wloka C, et al. Evidence that a septin diffusion barrier is dispensable for cytokinesis in budding yeast. Biol Chem. 2011;392:813–829. doi: 10.1515/bc.2011.083. [DOI] [PubMed] [Google Scholar]
- Wu SZ, Bezanilla M. Myosin VIII associates with microtubule ends and together with actin plays a role in guiding plant cell division. eLife. 2014;3:e03498. doi: 10.7554/eLife.03498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J-Q, Kuhn JR, Kovar DR, Pollard TD. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev Cell. 2003;5:723–734. doi: 10.1016/S1534-5807(03)00324-1. [DOI] [PubMed] [Google Scholar]
- Wu H, Guo J, Zhou YT, Gao XD. The anillin-related region of Bud4 is the major functional determinant for Bud4’s function in septin organization during bud growth and axial bud site selection in budding yeast. Eukaryot Cell. 2015;14:241–251. doi: 10.1128/ec.00268-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Carvalho P, Voeltz GK. Here, there, and everywhere: the importance of ER membrane contact sites. Science. 2018;361:eaan5835. doi: 10.1126/science.aan5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Vogel BE. A secreted protein promotes cleavage furrow maturation during cytokinesis. Curr Biol. 2011;21:114–119. doi: 10.1016/j.cub.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida M. Fission yeast cut mutations revisited: control of anaphase. Trends Cell Biol. 1998;8:144–149. doi: 10.1016/S0962-8924(98)01236-7. [DOI] [PubMed] [Google Scholar]
- Yang X, et al. Syntaxin opening by the MUN domain underlies the function of Munc13 in synaptic-vesicle priming. Nat Struct Mol Biol. 2015;22:547–554. doi: 10.1038/nsmb.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Lee I-J, Runge KW, Wu J-Q (2012) Roles of putative Rho-GEF Gef2 in division-site positioning and contractile-ring function in fission yeast cytokinesis. Mol Biol Cell 23:1181–1195. 10.1091/mbc.E11-09-0800 [DOI] [PMC free article] [PubMed]
- Yoon T-Y, Munson M. SNARE complex assembly and disassembly. Curr Biol. 2018;28:R397–R401. doi: 10.1016/j.cub.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Yu I-M, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Annu Rev Cell Dev Biol. 2010;26:137–156. doi: 10.1146/annurev.cellbio.042308.113327. [DOI] [PubMed] [Google Scholar]
- Yue P, et al. Sec3 promotes the initial binary t-SNARE complex assembly and membrane fusion. Nat Commun. 2017;8:14236. doi: 10.1038/ncomms14236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahner JE, Harkins HA, Pringle JR. Genetic analysis of the bipolar pattern of bud site selection in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:1857–1870. doi: 10.1128/mcb.16.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang JH, Cavet G, Sabry JH, Wagner P, Moores SL, Spudich JA. On the role of myosin-II in cytokinesis: division of Dictyostelium cells under adhesive and nonadhesive conditions. Mol Biol Cell. 1997;8:2617–2629. doi: 10.1091/mbc.8.12.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Feng S, Wu B, Guo W. Polarized exocytosis. Cold Spring Harb Perspect Biol. 2017;9:a027870. doi: 10.1101/cshperspect.a027870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Robinson DN. Balance of actively generated contractile and resistive forces controls cytokinesis dynamics. PNAS. 2005;102:7186–7191. doi: 10.1073/pnas.0502545102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Michelot A, Koskela Essi V, Tkach V, Stamou D, Drubin David G, Lappalainen P. Membrane-sculpting BAR domains generate stable lipid microdomains. Cell Rep. 2013;4:1213–1223. doi: 10.1016/j.celrep.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Dong F, Rasul F, Yao X, Q-w J, Zheng F, Fu C. Septins regulate the equatorial dynamics of the separation initiation network kinase Sid2p and glucan synthases to ensure proper cytokinesis. FEBS J. 2018;285:2468–2480. doi: 10.1111/febs.14487. [DOI] [PubMed] [Google Scholar]
- Zhou M, Wang Y-L, Chang F. Distinct pathways for the early recruitment of myosin II and actin to the cytokinetic furrow. Mol Biol Cell. 2007;19:318–326. doi: 10.1091/mbc.e07-08-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, et al. The contractile ring coordinates curvature-dependent septum assembly during fission yeast cytokinesis. Mol Biol Cell. 2015;26:78–90. doi: 10.1091/mbc.e14-10-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y-H, Hyun J, Pan Y-Z, Hopper JE, Rizo J, Wu J-Q (2018) Roles of the fission yeast UNC-13/Munc13 protein Ync13 in late stages of cytokinesis. Mol Biol Cell 29:2259–2279. 10.1091/mbc.E18-04-0225 [DOI] [PMC free article] [PubMed]
- Zimmermann D, et al. Mechanoregulated inhibition of formin facilitates contractile actomyosin ring assembly. Nat Commun. 2017;8:703. doi: 10.1038/s41467-017-00445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorec R. SNARE-mediated vesicle navigation, vesicle anatomy and exocytotic fusion pore. Cell Calcium. 2018;73:53–54. doi: 10.1016/j.ceca.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Zumdieck A, Kruse K, Bringmann H, Hyman AA, Julicher F. Stress generation and filament turnover during actin ring constriction. PLoS One. 2007;2:e696. doi: 10.1371/journal.pone.0000696. [DOI] [PMC free article] [PubMed] [Google Scholar]