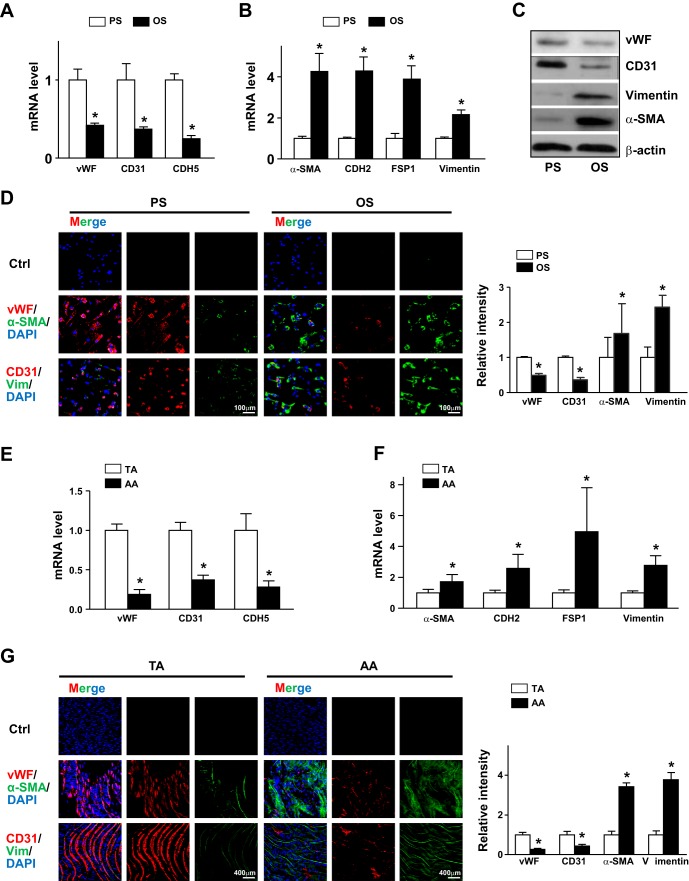
Fig. 2.
Pulsatile shear stress (PS) and oscillatory shear stress (OS) distinctly regulate the endothelial-to-mesenchymal transition in endothelial cells (ECs). A–D: human umbilical vein ECs were subjected to PS (12 ± 4 dyn/cm2) or OS (1 ± 4 dyn/cm2) for 24 h. mRNA levels of von Willebrand factor (vWF), CD31, caherin 5 (CDH5), α-smooth muscle actin (α-SMA), cadherin 2 (CDH2), fibroblast-specific protein 1 (FSP1), and vimentin (Vim) were measured by real-time quantitative PCR. C: protein levels of CD31, vWF, vimentin, and α-SMA were measured by Western blot analysis. The β-actin level was used as a loading control. D: immunofluorescence staining with anti-vWF (red), anti-CD31 (red), anti-α-SMA (green), and antivimentin (green) antibodies. E and F: intima were collected from aortic arch (AA) or thoracic aorta (TA) areas of C57BL/6 mice (n = 18). mRNA levels of the indicated genes were quantified by real-time quantitative PCR. G: en face immunofluorescence staining with anti-vWF (red), anti-CD31 (red), anti-α-SMA (green), and anti-vimentin (green) antibodies revealed the expression of EC and mesenchymal genes in TA or AA regions. In D and G, images were acquired and the fluorescent signal was analyzed by Nikon C2 confocal laser scanning microscopy. The fluorescence intensity of 100 randomly chosen cells was determined using ImageJ. Data are means ± SE from three independent experiments. *P < 0.05 between the indicated groups.