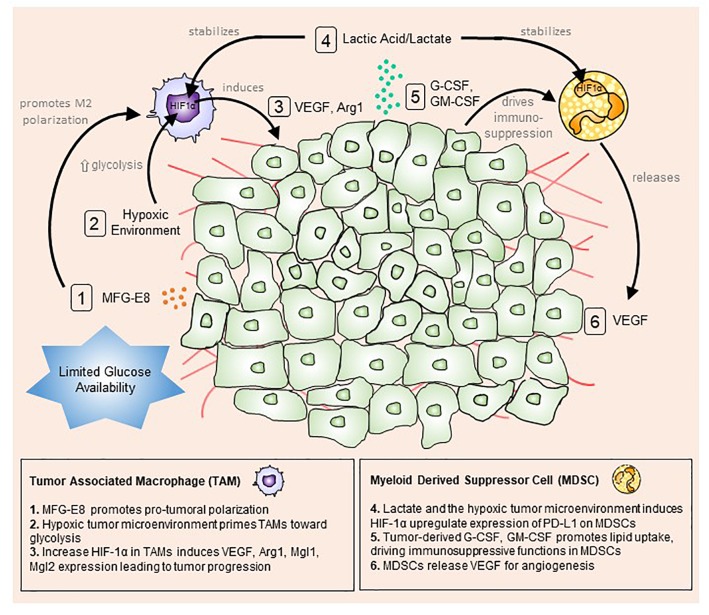
FIGURE 1.
The tumor microenvironment primes myeloid cells toward a pro-tumoral phenotype. Tumor cells actively uptake surrounding glucose to drive aerobic glycolysis and fuel their growth and proliferation. This mode of metabolism creates a microenvironment with limited available glucose and oxygen. Stressed tumor cells undergo apoptosis and produce milk fat globule-EGF factor 8 protein (MFG-E8), which promotes alternative (M2) macrophage polarization (1). At the same time, hypoxic conditions trigger macrophages to up-regulate hypoxia-inducible factor 1-alpha (HIF-1α), promoting a glycolytic switch (2). Lactic acid/lactate, the by-product of glycolysis, stabilizes HIF1α in tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells MDSCs (4). Increased HIF1α expression in TAMs enhances vascular endothelial growth factor (VEGF) and arginase 1 (Arg1) expression and secretion, which feedbacks to tumor cells to boost tumor progression (3). Conversely, tumor-derived granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) up-regulate lipid transport receptors to increase lipid metabolism and drive immunosuppressive functions in MDSCs (5). In turn, MDSCs release VEGF and cathepsin to induce angiogenesis and vasculogenesis (6).