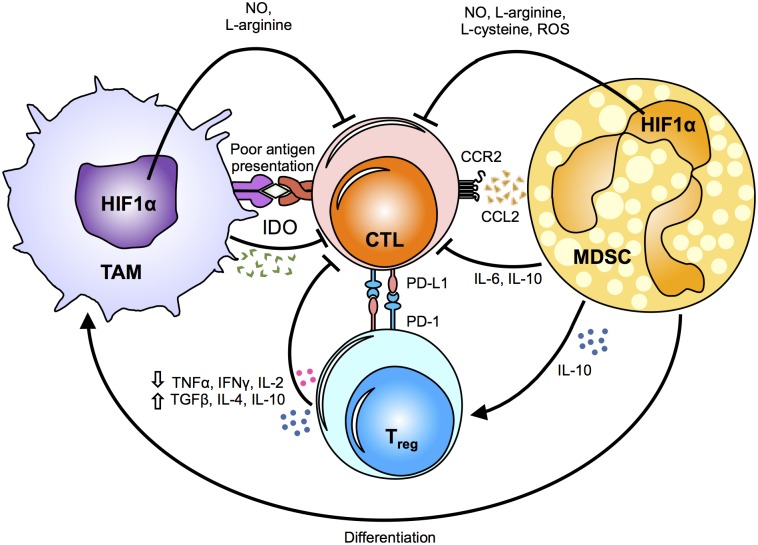
FIGURE 2.
Mechanisms of myeloid cell suppression in the tumor. The tumor microenvironment primes myeloid cells, such as tumor-associated macrophages (TAMs) and myeloid derived suppressor cells (MDSCs), by driving their function towards a pro-tumoral phenotype. The metabolic fates and alterations in myeloid cells caused by changes in the hypoxic tumor microenvironment induce the release of metabolic intermediates, such as nitric oxide (NO), L-arginine, L-cysteine and reactive oxygen species (ROS), which in turn modulate cytotoxic T-lymphocyte (CTL) effector responses. Notably, indoleamine 2,3-dioxygenase (IDO) secretion suppresses antigen-specific T-cell expansion. Chemokines and cytokines secreted by TAMs and MSDCs directly and/or indirectly inhibit the anti-tumoral cytotoxic responses of CTLs by inducing regulatory T cells (Tregs). In addition, PD-1 up-regulation on Tregs renders them as highly immunosuppressive cells. The critical role of TAMs and MDSCs in suppressing the anti-tumoral responses of CTLs supports that changes in their metabolic profile can influence the release of various cytokines and metabolic intermediates, and ultimately affect tumor growth, metastasis and drug resistance. HIF1α, hypoxia-inducible factor 1-alpha; TNFα, tumor necrosis factor alpha; IFNγ, Interferon gamma; IL, interleukin; TGFβ, transforming growth factor beta; CCR2, C-C chemokine receptor type 2; CCL2, chemokine (C-C motif) ligand 2; PD-L1, programmed death-ligand 1; PD-1, programmed cell death protein 1.