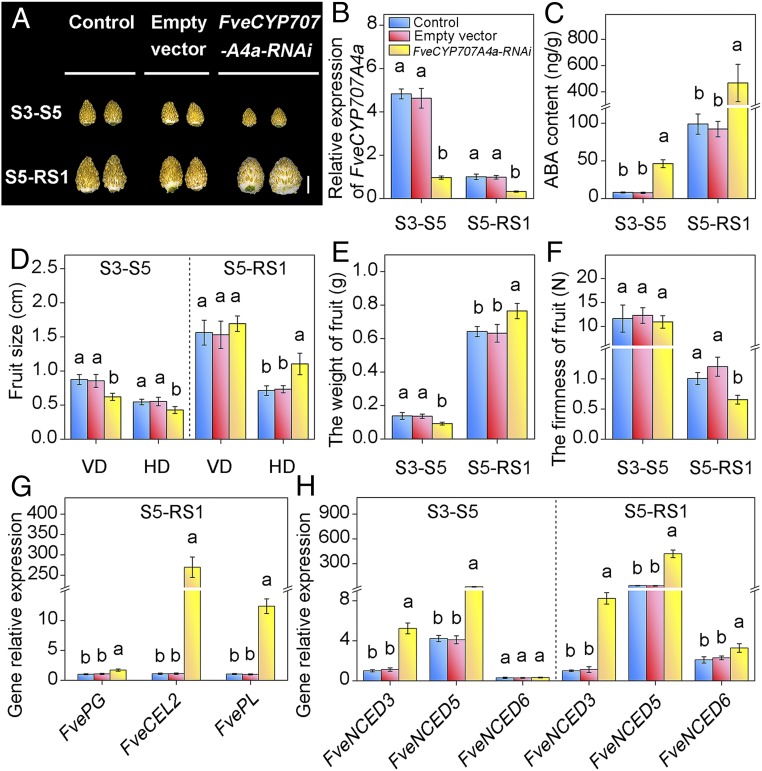
Fig. 4.
Transient silencing of FveCYP707A4a reveals dual function of ABA catabolism in fruit growth and ripening. (A) FveCYP707A4aRNAi S3–S5 inhibited fruit growth, while the FveCYP707A4aRNAi S5–RS1 promoted fruit ripening. FveCYP707A4aRNAi construct and the empty vector were injected into the fruits at 5 DAP (for FveCYP707A4aRNAi S3–S5) and 13 DAP (for FveCYP707A4aRNAi S5–RS1), respectively. Photos were taken 7 d after injection. (Scale bar: 5 mm.) (B) qRT-PCR analysis of transcript levels for FveCYP707A4a in FveCYP707A4aRNAi S3–S5 and FveCYP707A4aRNAi S5-RS1 fruits. Error bars represent SD of three independent replicates (15–20 fruits were used for each replicate). (C) FveCYP707A4a on ABA contents. Error bars represent SD of three independent replicates (10 fruits were used for each replicate). (D–F) The effect of transient silencing of Fve CYP707A4a on fruit (receptacle and achene) size (D), fresh weight (E), and firmness (F). Error bars represent SD of three independent replicates (15–20 fruits were used for each replicate). (G and H) qRT-PCR analysis of transcript levels for ripening-related genes (G) and FveNCED genes (H) in FveCYP707A4aRNAi S3–S5 and FveCYP707A4aRNAi S5–RS1 fruits. Error bars represent SD of three independent replicates. FveACTIN was used as the internal control for qRT-PCR analysis. Letter in figure indicates significant differences between groups (P < 0.05, one-way ANOVA, Tukey’s HSD post hoc test).