Fig. 3.
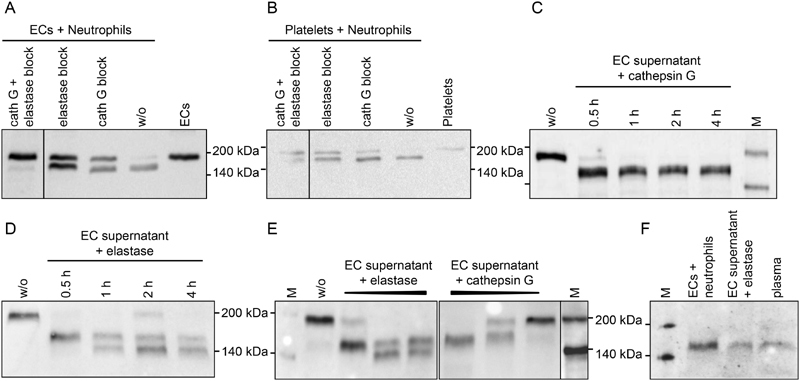
Thrombospondin-1 (TSP-1) processing by neutrophil-derived proteases. Freshly isolated human neutrophils were added to ( A ) confluent, serum-free cultures of endothelial cells or ( B ) platelets isolated from the same donor and incubated for 30 minutes at 37°C in the absence or presence of elastase inhibitor II (2.1 mM) and/or cathepsin G inhibitor I (0.1 mM). ( C–E ) Serum-free endothelial cell (EC) supernatant containing 185 kDa TSP-1 (w/o) was incubated with the purified proteases ( C ) cathepsin G at 10 mU/mL or ( D ) elastase at 20 mU/mL for 30 minutes to 4 hours, or ( E ) was exposed to increasing concentrations of elastase (10–50 mU/mL) and cathepsin G (2–50 mU/mL) for 30 minutes at 37°C. ( F ) The 160-kDa TSP-1 proteins generated either by co-culture of ECs with neutrophils or by 30-minute elastase digest of EC supernatant were compared with the smaller TSP-1 isoform prevalent in human plasma post-surgery. TSP-1 protein was detected in supernatants by immunoblotting with Ab11. Experiments were performed at least three times with blood from different donors. Please refer to Supplementary Fig. S3 (available in the online version) for quantitation of immunoblots. M, biotinylated protein marker.
