Fig. 4.
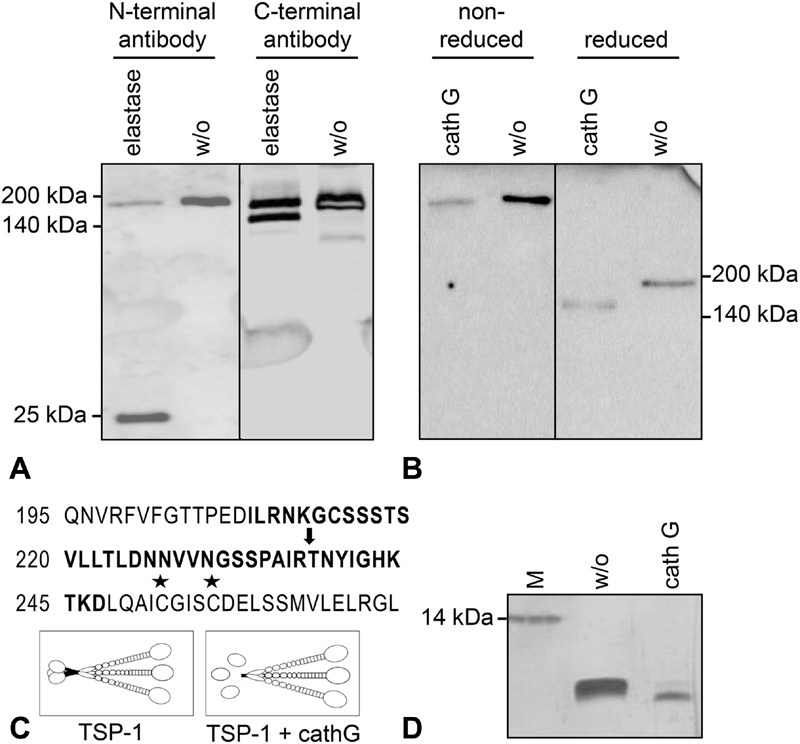
Molecular characterization of the 160-kDa thrombospondin-1 (TSP-1) isoform. Serum-free endothelial cell (EC) supernatant was either left untreated (w/o) or was digested with elastase (20 mU/mL) or cathepsin G (10 mU/mL) for 30 minutes at 37°C. ( A ) TSP-1 was detected in reduced protein samples by immunoblotting with two distinct antibodies, specific for the N-terminal or the C-terminal domain of TSP-1. ( B ) Reduced and non-reduced protein samples were compared on a gradient gel (4–20%) and TSP-1 was immunostained with Ab11, a combination of three monoclonal antibodies covering N- and C-terminal epitopes. ( C ) Illustration of the cathepsin G cut site (arrow) in the TSP-1 protein sequence as determined by mass spectrometry and Edman sequencing refers to amino acid numbering of secreted TSP-1 (not including the signal peptide); asterisks mark the cysteines involved in inter-chain disulphide bonds for trimerization. TSP-1 cleavage by cathepsin G results in the release of the monomeric N-terminal HBD (25 kDa) and a trimeric C-terminal core fragment of 160 kDa chains. ( D ) Silver staining of a synthetic peptide (comprising TSP-1 amino acids 208–247, bold letters in panel C) without or with digest by cathepsin G (50 mU/mL for 45 minutes).
