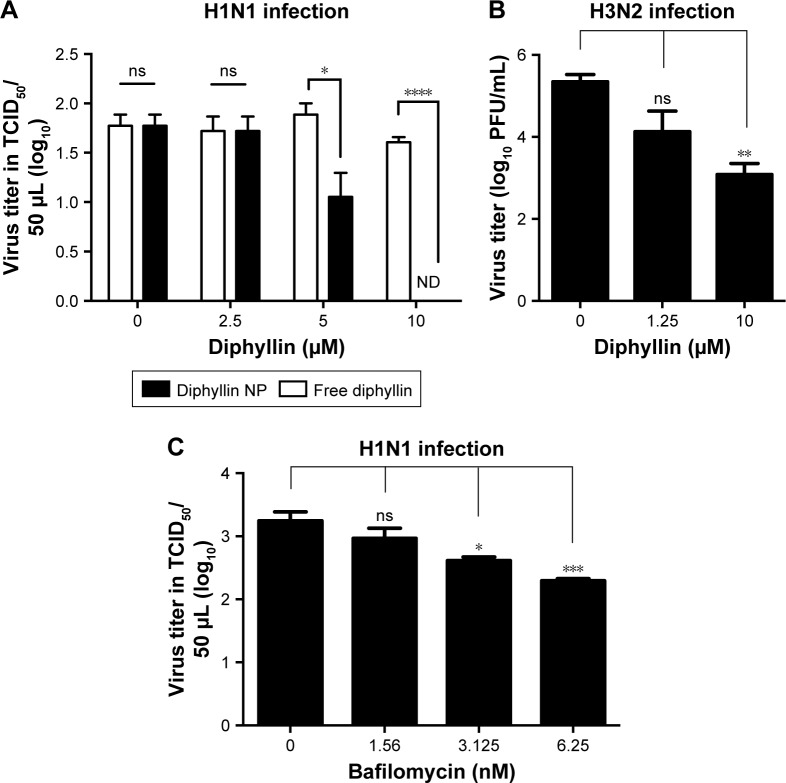
Figure 5.
Antiviral activity of free and nanoparticulate V-ATPase inhibitors.
Notes: (A) MH-S cells were pretreated with free diphyllin or diphyllin nanoparticles (diphyllin NP) and were infected with H1N1 virus at an MOI of 1. Following 24 hours of incubation, the supernatants were harvested to assess the viral TCID50 titers. Viral titers between free diphyllin and diphyllin NP-treated groups were compared by unpaired t-test. (B) MH-S cells were pretreated with diphyllin nanoparticles and were infected with H3N2 virus at an MOI of 5. Following 24 hours of incubation, the supernatants were harvested to assess virus titers by plaque assay. (C) MH-S cells were pretreated with bafilomycin nanoparticles and were infected with H1N1 at an MOI of 1. Following 24 hours of incubation, the supernatants were harvested to assess the viral TCID50 titers. Viral titers among drug-treated groups and the untreated control group were compared by one-way ANOVA followed by Dunnett’s multiple comparisons test (ns: nonsignificant, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001). Data in the plot represent mean ± SEM out of three replicates.
Abbreviations: V-ATPase, vacuolar ATPase; NP, nanoparticle; MOI, multiplicity of infection; TCID50, 50% tissue culture infective dose; SEM, standard error of the mean.