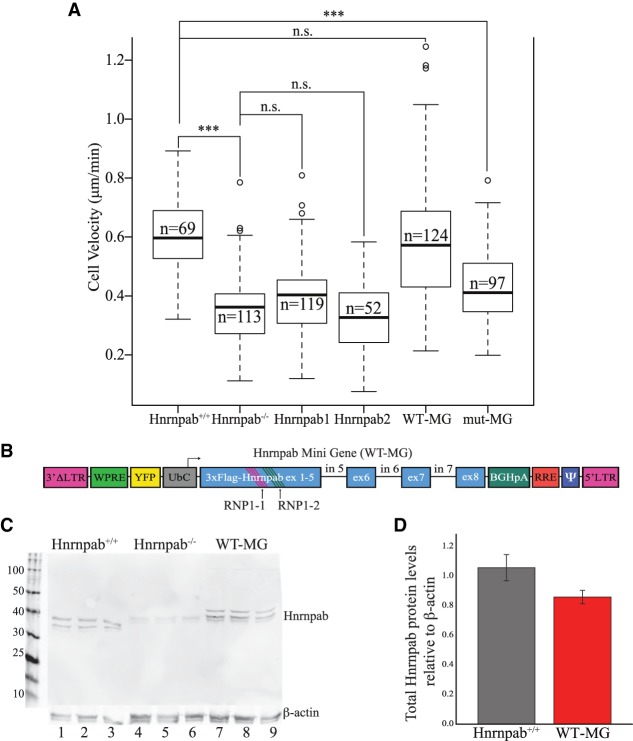
FIGURE 2.
Hnrnpab1 and Hnrnpab2 promote INC cell motility through the RRMs. (A) INC monolayers were scratched and imaged live as they filled in the wound. The velocity of cells was measured from these images and the results presented in box and whisker plots. The leftmost experiments were in Hnrnpab+/+ INCs, while all the other experiments were performed in Hnrnpab−/− INCs, with the reexpression of Hnrnpab isoforms as indicated below. P-values were calculated by one-way ANOVA followed by pairwise t-test with Bonferroni correction. (***) P < 0.001. (B) Diagram of lentiviral vector cassette to reexpress Hnrnpab minigene (WT-MG) and Hnrnpab mutant minigene (mut-MG). The first four exons of Hnrnpab cDNA were joined to exons 5–8 of Hnrnpab genomic DNA to facilitate alternative splicing of the recombinant protein cassette. The Hnrnpab MG lies in the inverse orientation of the lentiviral 5′ LTR promoter to retain Hnrnpab introns in the proviral genome. Two conserved phenylalanines in the RNP1 motifs of RRM1 (RNP1-1) and RRM2 (RNP1-2) were mutated to valine to create mut-MG. (C) Western blot showing expression of both Hnrnpab isoforms from WT-MG. Protein extracted from three independent cultures were run in parallel from either WT INCs (lanes 1–3), Hnrnpab−/− INCs (lanes 4–6), or Hnrnpab−/− INCs stably expressing WT-MG. Blots were probed with an antibody against endogenous Hnrnpab. The molecular weights of Hnrnpab1 and Hnrnpab2 from WT-MG are slightly higher than endogenous Hnrnpab1 and Hnrnpab2 due to 3xFlag tag. (D) The blots from panel C were quantified using Actb as a loading control, and the relative signal of Hnrnpab from WT INCs (gray bar) and Hnrnpab−/− INCs with WT-MG after Hnrnpab−/− background subtraction are shown. Error bars represent standard error.