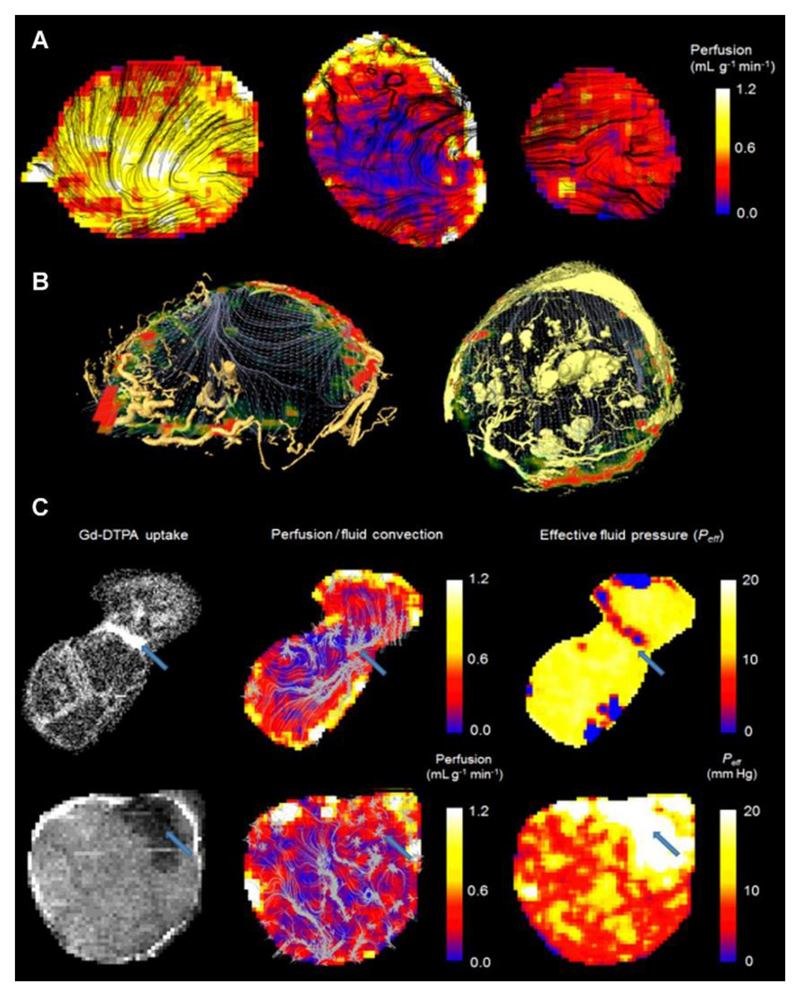
Figure 6.
Comparison of perfusion (measured using arterial spin labelling) and convection-MRI measurements. (a) Perfusion maps in SW1222 tumors (left and right) and an LS174T tumor (centre). ASL measurements are shown as heatmaps, and are overlaid with black streamlines showing the measured path followed by fluid within tumors. (b) Fluid flow measured in vivo with convectionMRI are shown as grey streamlines and perfusion is shown as a colorscale. The location of blood vessels is represented by yellow volume renderings, acquired using ex vivo microvascular casting and imaged with micro-CT. Data are shown in example LS174T (left) and SW1222 (right) tumors. In the SW1222 tumor, larger vessel structures in the centre of the tumor could be due to swelling by the casting material, although it is unclear if these vessels would have also been swollen under normal physiological conditions. A high-resolution version is provided in Supplemental Figure 3. (c) The relationship between fluid flow, vascular perfusion and the delivery of a medium molecular weight contrast agent in two LS174T tumors. Left column: Uptake of Gd-DTPA, an MRI contrast agent; middle column: vascular perfusion maps (color scale) overlaid with interstitial convection streamlines (grey); right column: effective pressure measurements from fluid mechanical modelling of convection-MRI data. The blue arrow in the top row shows a region between two lobes of the tumor in which the contrast agent preferentially accumulates, interstitial convection streamlines converge, a limited vascular supply is evident, and has a low IFP. In the bottom row, a blue arrow highlights a region with limited contrast agent uptake, a limited vascular supply, and with raised IFP. Both examples show the ability of convection-MRI and ASL, in combination, to identify regions that preferentially accumulate or resist the accumulation of exogenously administered agents. This could potentially be extended to the prediction of the uptake of therapeutic agents.