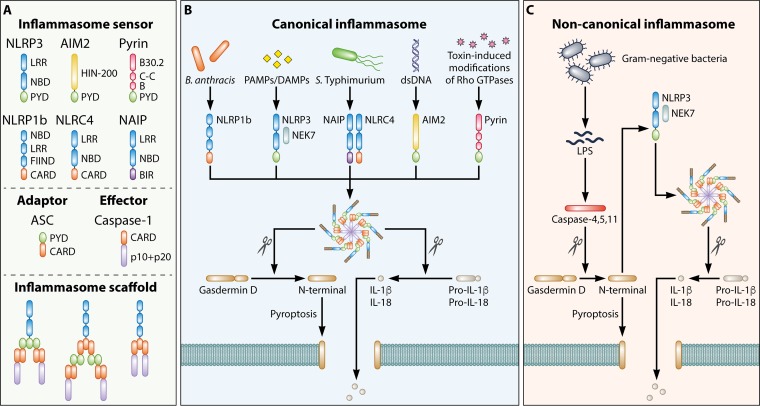
FIG 1.
Architecture of inflammasome complexes. (A) Formation of an inflammasome complex is initiated by an inflammasome sensor. Inflammasome sensors carry a pyrin domain (PYD) and/or a caspase activation and recruitment domain (CARD). They may also carry a leucine-rich-repeat domain (LRR), a nucleotide-binding domain (NBD), a HIN-200 domain, a B30.2 domain, a coiled-coil domain (C-C), a B-box domain (B), a function-to-find domain (FIIND), or a baculovirus inhibitor of apoptosis repeat (BIR). Other inflammasome components include the inflammasome adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC) and the effector protein caspase-1. A PYD-containing inflammasome sensor interacts with the PYD of ASC, allowing the CARD of ASC to interact with the CARD of caspase-1. A CARD-containing inflammasome sensor can interact with the CARD of ASC, whereby the PYD of ASC interacts with the PYD of an additional ASC. The CARD of ASC then interacts with the CARD of caspase-1. Alternatively, a CARD-containing inflammasome sensor may directly interact with caspase-1 via their respective CARDs. (B) Canonical inflammasome complexes are activated by a range of pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs). Caspase-1 cleaves the pore-forming factor gasdermin D, whereby the N-terminal domain of gasdermin D forms pores in the host cell membrane. Caspase-1 also cleaves the proinflammatory cytokines pro-IL-1β and pro-IL-18, generating biologically active versions of these cytokines for release through the membrane pores generated by gasdermin D. The pores formed by gasdermin D also lead to lytic cell death via pyroptosis. (C) The noncanonical inflammasome is a pathway specifically activated by Gram-negative bacteria. In this pathway, lipopolysaccharides (LPS) are introduced into the cytoplasm during infection and sensed by human caspase-4 and caspase-5 and mouse caspase-11. These inflammatory caspases can also cleave gasdermin D, in a manner similar to that by caspase-1, leading to the induction of pyroptosis. The N-terminal domain of gasdermin D also induces activation of the NLRP3 inflammasome and the associated proteolytic cleavage of pro-IL-1β and pro-IL-18.