Abstract
Background:
Circulating uric acid (UA) is an important biomarker, not only in the detection and management of gout, but also in assessing the risk of related comorbidity. The impact of collection methods on clinical UA measurements has been the subject of limited study. After observing significant differences between UA concentrations of blood samples obtained by different collection tubes, we began examining the effects of exogenous tube components on measured UA concentrations. We aimed to: (1) demonstrate the variability in uricase-based UA measurements attributable to different collection methods and (2) identify factors influencing this variability.
Methods:
Blood samples from human subjects were collected using Serum Separator Tubes (SST tubes), Acid Citrate Dextrose (ACD) tubes, and Sodium Citrate (SC) tubes. Circulating UA concentrations were measured by chemistry analyzers utilizing the uricase method. Absorbance assays were run in order to determine the effects of citric acid, sodium citrate, and dextrose on measured absorbance in the presence of leuco crystal violet dye, hydrogen peroxide, and peroxidase. Statistical analyses—including Student’s T tests and ANOVA—were used to compare results.
Results:
UA concentrations of blood samples collected in ACD tubes were significantly lower than those collected in SST tubes (P<0.01). Samples collected in SC tubes trended towards lower UA measurements than samples collected in SST tubes, although this difference did not reach statistical significance (P=0.06). Blood samples spiked with separate concentrations of sodium citrate (3.2 and 22.0 g/L), citric acid (8.0 g/L), and dextrose (24.5 g/L) demonstrated significantly lower UA measurements compared to controls (P<0.01). Absorbance assays demonstrated that increasing concentrations of citric acid and sodium citrate—in the presence of leuco crystal violet, hydrogen peroxide, and peroxidase—decreased the amount of oxidized dye in the uricase method of UA measurement in a dose-dependent manner (P<0.01). In contrast, dextrose did not significantly alter the amount of oxidized dye available.
Discussion:
Our results indicate that citric acid obstructs accurate uricase-based UA measurement, providing falsely low values. Citric acid, a known antioxidant, scavenges hydrogen peroxide, a key intermediate using the uricase method. By scavenging hydrogen peroxide, citric acid decreases the amount of oxidized leuco dye leading to falsely low UA measurements. Therefore, collection tubes, like ACD and SC tubes, which contain concentrations of citric acid or its conjugate base sodium citrate should not be used to measure circulating UA levels when utilizing uricase-based measurement methods.
Keywords: Uric acid measurement, uricase method, citric acid, sodium citrate, hydrogen peroxide, antioxidant
1. Introduction
Uric acid (UA), a commonly measured circulating metabolite of xanthine, is the end product of purine degradation in humans. Abnormal circulating UA concentrations are implicated in the pathogenesis of many clinical diagnoses, most notably serving as an important biomarker in the detection and management of gout [1]. Gout—the most common inflammatory arthritis—is characterized by intermittent pain and inflammation of joints resulting from hyperuricemia (commonly defined as blood urate concentrations in excess of 6.8 mg/dL) and the articular/periarticular deposition of insoluble monosodium urate crystals [2]. The accurate measurement of circulating UA may also inform the management of patients with other comorbid conditions, as hyperuricemia is associated with higher rates of hypertension, hyperlipidemia, renal failure, cardiovascular disease, diabetes mellitus, and obesity [3, 4]. Additionally, proper measurement of UA in bio-banked serum or plasma is crucial to understanding the pathogenesis and epidemiological characteristics of UA-mediated diseases.
Recent investigations have focused on the long-term stability of metabolites in blood samples, as well as differences in metabolite concentrations based upon blood sample type [5–8]. Liu and Aa et al. demonstrated that UA concentrations measured from plasma ethylenediaminetetraacetic acid (EDTA) samples were significantly higher than serum samples using gas chromatography and time-of-flight mass spectrometry [6]. In contrast, Barri et al. used mass spectrometry to demonstrate lower UA measurements of plasma EDTA and sodium citrate samples compared to serum that was dependent upon the charge of the UA molecule [7]. Liao et al. compared UA measurements utilizing an electrochemical detection device that used an electrode to oxidize UA, producing a measurable current proportional to UA concentration. Their study found that blood samples collected into a sodium citrate anticoagulant manifested 10.4% lower UA measurements as compared to a standard reference [8]. Further research has yielded conflicting results regarding the measurement of metabolites such as UA based on differences in sample collection and the assay used [9–12].
While mass spectrometry and electrochemical detection devices can accurately measure circulating UA concentrations in blood samples regardless of citric acid, they are not commonly utilized by clinical laboratories as part of routine patient care due to the high cost and time. In fact, little research has been dedicated to examining differences in UA concentrations between sample types using clinically-relevant measurement methods. Our present research was performed after observing marked differences between UA concentrations measured from blood samples procured using Acid Citrate Dextrose (ACD) tubes—collection tubes regularly used to gather plasma samples for biobanking studies— and Serum Separator Tubes (SST tubes). One of the limitations of the uricase base method is that it produces hydrogen peroxide, which is further utilized by the assay as a direct proportional color change detectable by reflected light. Any substance capable of scavenging hydrogen peroxide will cause a decrease in the color change, rendering this method in valid. In our study, UA concentrations were measured using chemical analyzers that leveraged the uricase reaction, the most common method used by clinical laboratories to measure UA. Based on these findings and clinical importance of UA measurements, we aimed to examine the impact of ACD tubes and its separate components on UA concentrations measured by the uricase method. The objective of our present research was to: (1) demonstrate variability in uricase-based UA measurement attributable to different collection methods and (2) identify factors underpinning any observed variability.
2. Materials and Methods
2.1. Materials
Uric acid (≥99.0%, crystalline), citric acid monohydrate (≥99.0%, reagent grade), D-(+)-glucose (dextrose) (≥99.5%), leucocrystal violet dye, horseradish peroxidase (lyophilized solid), hydrogen peroxide solution (30.0% [w/w] in H2O), and potassium phosphate monobasic (≥99.0%) were purchased from Sigma-Aldrich (St. Louis, MO). Sodium citrate (≥99.0%, anhydrous) was purchased from Alfa Aesar (Tewksbury, MA). Blood samples were collected using SST tubes, ACD Solution A tubes, and/or Sodium Citrate (SC) tubes obtained from Becton Dickinson Biosciences (Franklin Lakes, NJ). SST tubes contain a clot activator and gel for efficient serum separation from other blood components. We used ACD solution A tubes containing 22.0 g/L sodium citrate anticoagulant, 24.5 g/L dextrose for preservation of collected samples, and 8.0 g/L citric acid to prevent oxidation of dextrose. SC tubes hold buffered 3.2 g/L sodium citrate solution for anticoagulation purposes.
2.2. Collection of blood samples from VARA cohort and subcohort
UA concentrations from samples collected by SST tubes (SSTua) and ACD tubes (ACDua) were measured in patients enrolled in the Veteran’s Affairs Rheumatoid Arthritis (VARA) registry (n=2,399) by the Omaha Veteran’s Affairs Medical Center clinical laboratory (Lab A) as part of a separate study examining associations of circulating urate levels with disease outcomes. VARA participants provided informed consent for the collection of blood for biobanking purposes. This study was approved by the Omaha VA Institutional Review Board and the VARA Scientific Ethics Advisory Committee. Details regarding the VARA registry are described elsewhere [13].
VARA blood samples, collected over a 14 year span, were drawn in ACD and SST tubes and stored at −70 °C until analysis. All samples were centrifuged at 2,000 RPM for 10 minutes and the serum and plasma supernatants were obtained for metabolite analysis. Convenience samples from thirty patients within the VARA cohort were selected representing low (1.0–3.0 mg/dL UA), intermediate (3.1–5.0 mg/dL UA), and high (5.1–7.0 mg/dL UA) ranges of ACDua as measured by Lab A (n=10 from each range). SSTua and ACDua from this VARA subcohort were then measured by a clinical laboratory based at the University of Nebraska Medical Center (Lab B) in order to determine if significant differences existed between lab values (see UA measurement below). Results generated using banked samples from the VARA subcohort were compared to UA concentrations ascertained for the same individuals as part routine care that were available within the medical record (available for 15 of 30 patients). These blood samples were collected as part of routine care received at the Omaha Veteran’s Affairs Medical Center using Greiner Bio-One Lithium Heparin (LH) Tubes (Kremsmünster, Upper Austria) that contain lithium heparin anticoagulant. UA values collected in LH tubes as part of routine care (LHua) were measured in Lab A.
2.3. Collection of blood samples from healthy volunteers
Following analyses of the VARA subcohort, an initial blood sample was collected in a falcon plastic tube, SST, ACD, sodium citrate, EDTA, and lithium heparin tubes, from one healthy volunteer to determine if other methods of blood collection effect UA values. Results obtained indicated that only tubes containing citrate effected the values. Therefore, EDTA and lithium heparin tubes were not evaluated further in the study. We next collected blood samples from three healthy volunteers. Given the significant differences in UA concentrations based on sample type, we examined the effects of relevant components of ACD tubes—citric acid, sodium citrate, and dextrose—that might interfere with UA measurement given results from the VARA cohorts. We expanded our analysis to include SC tubes due to the presence of sodium citrate anticoagulant that may also interfere with UA measurement based on acid-base equilibrium. Blood from the healthy volunteers was collected into control SST tubes, SC tubes, and ACD tubes. Samples were also collected into control 15 ml tubes containing 1 ml 10X phosphate buffered saline (PBS) with no additives, as well as separate tubes with 22.0 g/L sodium citrate, with 3.2 g/L sodium citrate, with 8.0 g/L citric acid, and with 24.5 g/L dextrose in order to mimic the concentrations of relevant components in collection tubes. All additives were suspended in 1 ml 10X PBS before collecting blood samples. Approximately 5mL of blood was collected in each tube. All samples were centrifuged at 2,000 RPM for 10 minutes and the supernatant was obtained for UA analysis. UA concentrations of healthy volunteer samples were measured by Lab A.
2.4. UA measurement methods
Lab A used the VITROS URIC Slide Chemistry method (Ortho Clinical Diagnosis, Raritan, NJ), which uses the uricase reaction (Scheme 1A). Using this method, the patient’s blood sample is added to a VITROS URIC slide and is evenly spread to the underlying reagent layer. In the reagent layer, UA is oxidized to allantoin and hydrogen peroxide by uricase. In the presence of hydrogen peroxide, horseradish peroxidase oxidizes 2-(3,5-dimethoxy-4-hydroxyphenyl)-4,5-bis-(4-dimethylaminophenyl) imidazole (leuco dye) to form a colored dye that is analyzed by a colorimetric analyzer to generate a UA value based on the amount of dye produced. Lab B used the Beckman Coulter AU 5800 analyzer (Beckman Coulter, Brea, CA), which utilizes the uricase reaction in the manner outlined in Scheme 1B. Similar to the method used by Lab A, UA is oxidized to allantoin and hydrogen peroxide by the uricase enzyme. Hydrogen peroxide then reacts with 4-aminoantipyrine (4-APP) and N,N-bis(4-sufobutyl)-3,5-dimethylaniline, disodium salt (MADB) in the presence of horseradish peroxidase to produce a chromophore that is measured by colorimetry to produce a UA value. The Uricase enzyme and other reagents (when opened) used in this test are stable on the analyzer for less than 2 hours when turned off and less than 2 weeks when the machine is turned on.
Scheme 1. Schematic diagram of uricase-based methods to measure uric acid concentrations in Lab A and Lab B.

(A) Reaction utilized by VITROS URIC Slide Chemistry method to measure UA concentration by Lab A. The first part of the reaction involves the conversion of UA to allantoin, carbon dioxide, and hydrogen peroxide in the presence of uricase enzyme. In the latter half of the uricase method involving the presence of hydrogen peroxide, peroxidase oxidizes the leuco dye to produce a colorimetric dye that is proportional the concentration of UA in the sample. (B) Reaction utilized by the Beckman Coulter AU 5800 analyzer in Lab B. Similar to Lab A, the first half of the reaction utilizes uricase to convert UA to allantoin, carbon dioxide, and hydrogen peroxide. Additionally, the second part of the reaction involves the action of peroxidase to indirectly measure UA concentrations by oxidizing a dye in the presence of hydrogen peroxide.
Figure 1. A) The reaction outlined was utilized by VITROS URIC Slide Chemistry method to measure uric acid concentration by Lab A. Both uricase and peroxidase are the key enzymes involved in the reaction B) The reaction outlined was utilized by the Beckman Coulter AU 5800 analyzer in Lab B. Similar to Lab A, the reaction involves the enzymes uricase and peroxidase to indirectly measure uric acid concentrations.
2.5. Peroxidase activity absorbance assay
Absorbance assays were performed measuring peroxidase activity through a reduction-oxidation reaction that mimicked aspects of the uricase method utilized by clinical laboratories to measure circulating UA concentrations. We measured peroxidase activity through absorbance assays that quantified the amount of leuco dye oxidation by horseradish peroxidase in the presence of hydrogen peroxide. Oxidation of leuco dye produces a colored dye proportional to UA concentrations. Through measurement of absorbance changes in the peroxidase reaction, we set out to determine if citric acid, sodium citrate, and/or dextrose affected the amount of oxidized leuco dye, indicating interference with the uricase method.
Solutions of 0.1, 1.0, 2.0, 4.0, and 8.0 mg/mL citric acid were made using 100mM monopotassium phosphate buffer. Additionally, 1.0, 3.2, 10.0, 22.0, and 30.0 mg/mL sodium citrate solutions as well as 1.0, 5.0, 10.0, 24.5, and 40.0 g/L dextrose solutions were made using buffer. Horseradish peroxidase was prepared at 0.06 units, 1/10th the concentration of horseradish peroxidase used by Lab A and Lab B in the uricase method of UA measurement. A 5 mM stock solution of leuco dye was prepared by solubilizing leucocrystal violet in dimethyl sulfoxide. Using buffer and stock leucocrystal violet solution, a 1:1 mixture of 50 uM leucocrystal violet and 3% hydrogen peroxide solution was prepared to initiate the reaction. 50 uL of citric acid, sodium citrate, or dextrose solutions at the indicated concentrations were added to sample wells of a clear 96-well plate. 50 uL of horseradish peroxidase stock solution was also added to each sample well. To initiate the reaction, 50 uL leucocrystal violet/3% hydrogen peroxide (1:1) solution was added to each well. Following 20 minutes of incubation in the dark, absorbance was recorded at 590 nm using a BioTek Epoch Microplate Spectrophotometer (Biotek Instruments, Winooski, VT) and compared to controls. Absorbance values were normalized so that control samples averaged 1.0 in order to compare absorbance values across different plates.
2.6. Data processing and statistics
Student’s t-test as well as Tukey’s, Sidak’s, and Dunnett’s multiple comparison tests were utilized for comparisons as appropriate. ANOVA tests were used to determine statistical significance between multiple group means. Spearman’s rank correlation coefficient was utilized to determine whether there was an association between the calendar year of VARA sample procurement and ACDua value. ACDua and SSTua measurements were compared to lifetime average LHua measurements taken as part of routine care and abstracted from electronic health data available from 15 of the 30 VARA subcohort patients. UA concentrations of these patients were measured by Lab A. Pearson product-moment correlation was used to determine the correlation between sample types taken at different lab sites.
3. Results
3.1. Variability of UA measurements based on collection method
ACDua and SSTua were measured in biobanked samples from the VARA cohort (n=2,399) by Lab A as part of an investigative study on circulating urate levels. Mean ACDua was found to be 3.2 mg/dL (SD = 1.0), whereas mean SSTua measured was 4.9 mg/dL (SD = 2.1) (P<0.01) (Figure 1). These differences were not related to calendar year of procurement reflecting the duration of specimen banking (Spearman correlation =0.04, P=0.84). The differences observed between ACDua and SSTua values in the VARA cohort led to additional statistical and chemical analysis using the VARA subcohort (n=30), a convenience sample of the larger VARA cohort. The VARA subcohort was composed entirely of male subjects, who were primarily Caucasian (86%) with a mean age of 68 (SD = 10) years and a mean body mass index of 28 kg/m2 (SD = 5).
Figure 1. Differences between UA concentrations measured from SST tubes and ACD tubes in VARA cohort.
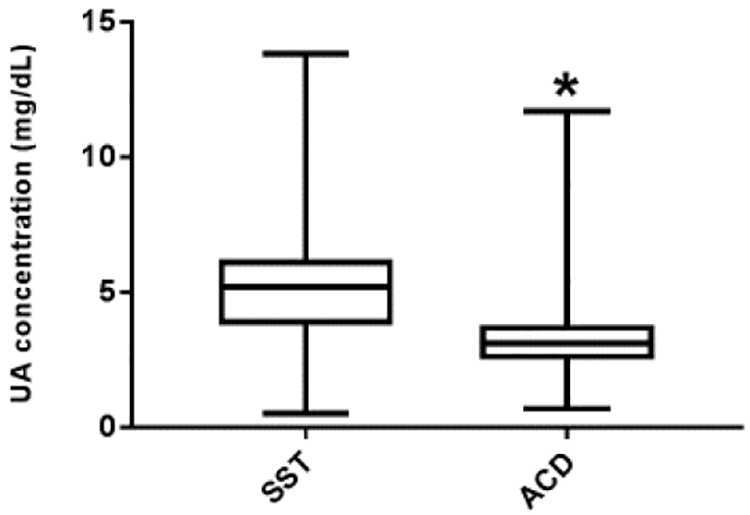
UA concentrations in blood samples procured with SST tubes and ACD tubes. Blood samples from VARA cohort (n=2,399) were collected into both SST tubes and ACD tubes between 2003 and 2017 and UA concentration was measured by Lab A. Statistically significant differences were observed between SSTua (μ=4.9 mg/dL, SD=2.1) and ACDua (μ=3.2 mg/dL, SD=1.9) (P<0.01). *indicates statistical significance at the 95% CI.
Results from Lab A demonstrated a mean ACDua in the VARA subcohort of 3.7 mg/dL (SD = 1.9) and a mean SSTua of 6.0 mg/dL (SD = 1.7) (P<0.01). We examined the reproducibility of the results by analyzing UA measurements of the VARA subcohort in a separate lab, Lab B, which utilized a similar uricase method as Lab A. Mean VARA subcohort ACDua measured by Lab B was 3.5 mg/dL (SD = 1.5) compared to a mean SSTua of 5.9 mg/dL (SD = 1.7) (P<0.01) SU values generated by the two laboratories appeared to be more strongly correlated for SSTua (RSSTua=0.98, μSSTua, P<0.01) than for ACDua (RACDua=0.73, P<0.01) (Figure 2). Likewise, the mean percent difference in ACDua vales generated by the two laboratories was 38.5% compared to a mean percent difference in SSTua values of just 4.9%. As a result, we suspected that ACD tubes were likely the source of variation in observed UA measurements. In order to confirm this, we compared SSTua and ACDua to UA results from clinically-derived samples that were collected in Lithium Heparin tubes (LHua) and abstracted from available medical records of 15 patients in the VARA subcohort. A ratio of LHua (the mean of all measurements available for a given patient) to Lab A SSTua and ACDua demonstrated that SSTua was strongly associated with lifetime mean LHua (LHua/SSTua =0.91, SD=0.27). In contrast, ACDua were nearly two times lower than available LHua for available patients (LHua/ACDua=1.80, SD=1.04). Therefore, we determined that blood collected in ACD tubes provided falsely low UA measurements.
Figure 2. Comparison of UA concentrations measured by Lab A and Lab B using blood samples from healthy volunteers.
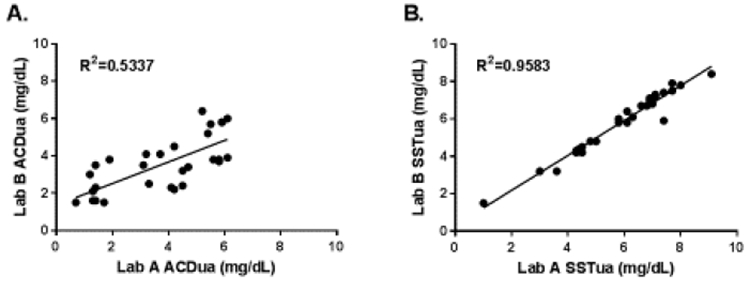
Pearson product-moment correlation was calculated to examine differences in UA concentrations measured by the two different laboratories. (A) Correlation of ACDua from the same blood sample measured by Lab A and Lab B (R2=0.5337, P<0.01). Mean percent difference between Lab A ACDua and Lab B ACDua was 38.5%. (B) Correlation of SSTua from the same blood sample measured by Lab A and Lab B (R2=0.9584, P<0.01). Mean percent difference between Lab A SSTua and Lab B SSTua was 4.9%.
3.2. Effects of exogenous components on UA measurement
Next, we aimed to determine the culprit(s) behind the skewed UA measurements. We examined the effects of exogenous tube components on UA measurement using three healthy volunteer subjects. Blood samples were procured using SST tubes (used to compare samples given strong association with lifetime mean LHua), SC tubes, and ACD tubes. Circulating UA concentrations were then measured by Lab A (Figure 3A). Mean control SSTua in healthy volunteers was 7.2 mg/dL (SD=0.90), whereas mean SC tube UA concentrations (SCua) and ACDua was 5.6 mg/dL (SD=0.6) and 4.3 mg/dL (SD=0.6), respectively. Statistically significant differences existed between mean SSTua and mean ACDua measured in the healthy volunteers (P<0.01). Differences between SSTua and SCua trended towards but did not reach statistical significance (P=0.06). Blood was also procured into tubes with no additives (acting as the control), 22.0 g/L sodium citrate, 3.2 g/L sodium citrate, 8.0 g/L citric acid, or 24.5 g/L dextrose added separately and tested for UA levels by Lab A (Figure 3B). UA concentrations measured using control tubes (μ=7.3 mg/dL, SD=0.9) did not significantly differ from SSTua concentrations in the healthy volunteers (P=0.42). Additionally, there was no statistically significant difference between UA concentrations of tubes with 3.2 g/L sodium citrate additive (μ=5.3 mg/dL, SD=0.7) and SC tubes (P=0.32). However, significant differences existed between control tubes and tubes with 3.2 g/L sodium citrate (P<0.01), 22.0 g/L sodium citrate (μ=3.7 mg/dL, SD=0.4, P<0.01), 8.0 g/L citric acid (μ=3.6 mg/dL, SD=0.5, P<0.01), and 24.5 dextrose (μ=4.1 mg/dL, SD=0.3, P<0.01), indicating that each of these components may be responsible for abnormally low UA measurements.
Figure 3. UA concentrations of healthy volunteers using various collection tubes and individual collection tube components.

UA concentrations in healthy volunteer blood samples procured by different collection methods. *indicates statistical significance at the 95% CI. (A) Sample collection in in commercial collection tubes. Significant differences existed between SSTua (μ=7.2 mg/dL, SD=0.9) and ACDua (μ=4.3 mg/dL, SD=0.6) (P<0.01). Differences approached significance between SSTua and SCua (μ=5.6 mg/dL, SD=0.6) (P=0.06). (B) Sample collection in tubes spiked with 3.2 g/L sodium citrate, 22.0 g/L sodium citrate, 8.0 g/L citric acid, and 24.5 g/L dextrose. Samples were compared to controls tubes with no additives. Significant differences existed between controls and 3.2 g/L sodium citrate (μ=5.3 mg/dL, SD=0.7, P<0.01), 22.0 g/L sodium citrate (μ=3.7 mg/dL, SD=0.4, P<0.01). 8.0 g/L citric acid (μ=3.6 mg/dL, SD=0.5, P<0.01), and 24.5 g/L dextrose (μ=4.1 mg/dL, SD=0.3, P<0.01).
To further determine the effects of tube components on the uricase reaction, we measured the absorbance of oxidized leuco dye in the presence of varying relevant concentrations of citric acid, sodium citrate, and dextrose (Figure 4). After normalization of values, mean absorbance values for samples with 0.1, 1.0, 2.0, 4.0, and 8.0 g/L citric acid added were 1.065 (SD=0.031), 0.809 (SD=0.133), 0.113 (SD=0.005), 0.084 (SD=0.003), and 0.084 (SD=0.003), respectively (Figure 4A). Statistically significant differences existed between control samples and 2.0 (P<0.01), 4.0 (P<0.01), and 8.0 g/L citric acid samples (P<0.01). In 1.0 g/L citric acid samples, differences trended towards significance (P=0.06). Mean absorbance values for samples spiked with 1.0, 3.2, 10.0, 22.0, and 30.0 g/L sodium citrate were 1.030 (SD=0.044), 1.027 (SD=0.058), 0.822 (SD=0.012), 0.652 (SD=0.054), and 0.598 (SD=0.047), respectively (Figure 4B). Compared to controls with no additives, statistically significant differences existed in 10.0 (P=0.02), 22.0 (P<0.01), and 30.0 g/L sodium citrate samples (P<0.01). Examination of samples spiked with 1.0, 5.0, 10.0, 24.5, and 40.0 g/L dextrose yielded absorbance values of 0.982 (SD=0.029), 1.020 (SD=0.050), 1.012 (SD=0.043), 1.173 (SD=0.111), and 1.141 (SD=0.240), respectively (Figure 4C). No statistically significant differences existed between any of the dextrose concentrations and control samples. Our results indicate that citric acid starts to inhibit peroxidase function at a concentration of 1.0 g/L and sodium citrate at 10.0 g/L , thereby, preventing oxidation of the leuco dye. Additionally, we determined that the effects of dextrose on UA measurement act in a manner independent of peroxidase function.
Figure 4. In vitro absorbance assay.
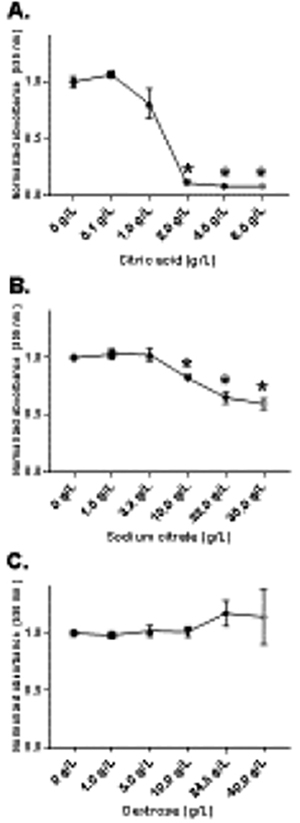
Absorbance values were measured by BioTek Epoch Microplate Spectrophotometer. Values were normalized so that control samples averaged an absorbance of 1.0. Varying concentrations of citric acid, sodium citrate, and dextrose were added to samples wells and compared to controls with no additives. *indicates statistical significance at the 95% CI. (A) Mean absorbance values for samples with 0.1, 1.0, 2.0, 4.0, and 8.0 g/L citric acid added were 1.065 (SD=0.031), 0.809 (SD=0.133), 0.113 (SD=0.005), 0.084 (SD=0.003), and 0.084 (SD=0.003), respectively. (B) Mean absorbance values for samples spiked with 1.0, 3.2, 10.0, 22.0, and 30.0 g/L sodium citrate were 1.030 (SD=0.044), 1.027 (SD=0.058), 0.822 (SD=0.012), 0.652 (SD=0.054), and 0.598 (SD=0.047), respectively. (C) Mean absorbance values for samples with 1.0, 5.0, 10.0, 24.5, and 40.0 g/L dextrose were 0.982 (SD=0.029), 1.020 (SD=0.050), 1.012 (SD=0.043), 1.173 (SD=0.111), and 1.141 (SD=0.240), respectively.
4. Discussion
To our knowledge, this is the first study to demonstrate marked variability between ACDua and SSTua concentrations using a uricase-based UA measurement method. This study adds to a number of prior reports examining the effects of sample procurement methods on UA measurement. We observed an approximately 1.5-fold difference between measured UA concentrations from SST tubes and ACD tubes across all cohorts that were examined. Our results show that when stored at −70oC the duration sample storage appears to have little effect on UA values. With results replicated in two separate laboratories (both using a similar uricase-based method common to clinical laboratories), we found that SSTua concentrations more closely resembled “true” lifetime average UA measurements using a well-defined cohort, implicating ACD tubes as a source of falsely low analyte levels. In addition to generating systematically lower values, visual inspection of the linear regression curves also indicated that ACDua concentrations varied far more between labs than SSTua. Furthermore, we found that blood samples collected in SC tubes had lower mean UA measurements than serum SST controls with differences that approached, but did not reach, statistical significance (likely owing to the small number of samples examined). In these experiments, we found no difference between SSTua and UA measured from control tubes (no additives present), results that were expected since each is considered a coagulated serum blood sample. Moreover, there was no differences between SCua and UA measurements from 3.2 g/L sodium citrate tubes since each collection method utilized the same concentration of sodium citrate for blocking coagulation. Additionally, we found that all relevant concentrations of citric acid, sodium citrate, and dextrose added to blood samples significantly decreased measured UA concentrations. Blood samples spiked with sodium citrate showed a concentration-dependent decrease in measured UA, with samples collected into 3.2 g/L sodium citrate decreasing UA concentrations by nearly 30% and samples collected into 22.0 g/L sodium citrate decreasing UA concentrations by nearly 50%.
As part of our detailed examination of the effects of exogenous tube components, we identified citric acid, a component of ACD tubes, as the likely culprit of these untoward effects. Citric acid is a known antioxidant that acts to inhibit the oxidation of other molecules by scavenging reactive oxygen species (ROS) that are produced as by-products of normal cellular function [14]. In addition to scavenging hydrogen peroxide, other ROS scavenged by citric acid include hydroxyl, alkoxy, and peroxide radicals, and superoxide anions. Antioxidants like citric acid stabilize ROS through the direct transfer of hydrogen atoms from the antioxidant molecule in order to prevent ROS-mediated cell damage [15–17]. In ACD tubes, citric acid is responsible for preventing the oxidation of dextrose, a key component of red blood cell preservation in banked samples. Sodium citrate, the component responsible for anticoagulation in ACD and SC tubes, is the conjugate base of citric acid. Therefore, sodium citrate solubilized in solution will accept protons that are donated by the buffer solution to form variable concentrations of citric acid based on the strength of the buffer. The uricase-method of UA measurement utilizes hydrogen peroxide as a key reaction intermediate in determining circulating UA concentrations in blood samples. The amount of hydrogen peroxide produced by the uricase enzyme is directly proportional to the concentration of UA in the sample. In the latter component of the uricase method, hydrogen peroxide is reduced in the presence of peroxidase, ultimately leading to the oxidation of the leuco dye into a chromophore that produces a UA value proportional to the amount of oxidized dye. We hypothesized that citric acid in ACD tubes, as well as the concentrations of citric acid in SC tubes, were stabilizing the hydrogen peroxide produced by the uricase method of UA measurement, ultimately leading to decreased availability of hydrogen peroxide for oxidation of the leuco dye by peroxidase, resulting in abnormally low UA measurements.
In order to test our hypothesis, we used a peroxidase activity assay that measured oxidized leucocrystal violet dye in the presence of hydrogen peroxide, horseradish peroxidase, and varying concentrations of citric acid, sodium citrate, and dextrose. Our results demonstrated significant differences in absorbance values in samples spiked with 2.0 g/L citric acid or more, confirming that citric acid interferes with the latter half of the uricase reaction. Additionally, samples with 10.0 g/L sodium citrate or more showed significantly decreased absorbance values compared to controls, although not at the level of citric acid. These results corroborated our hypothesis that the antioxidant properties of citric acid interfere with obtaining an accurate UA measurement. At concentrations of citric acid found in ACD tubes, absorbance values decreased by 91.6%. Furthermore, at concentrations of sodium citrate found in ACD tubes, the oxidation of the leuco dye decreased by 34.8%. These observed differences are likely the culprit behind skewed uricase-based UA measurements observed across all cohorts when ACD tubes were used.
Although blood samples spiked with dextrose also demonstrated lower UA values, we did not observe a significant difference in absorbance values for dextrose samples. These results indicate that dextrose may play a role in altering UA measurements, but in a mechanism independent of peroxidase activity. Dextrose is a highly oxidizable compound that may interfere with the uricase method. In fact, Cook et al. demonstrated that measured serum UA concentrations unexpectedly decreased in hyperglycemic patients whose glucose exceeded 180 mg/dL, approximately 8% of the D-(+)-glucose present in ACD Solution A tubes [18]. It is possible that non-physiologic concentrations of dextrose may have an inhibitory effect on the uricase method of UA measurement. Further examination of elevated glucose levels and their effects on measured UA concentrations may be highly clinically relevant, particularly in light of the disproportionately higher frequency of diabetes seen in patients with gout [3, 4].Our present research indicates that components within ACD and SC tubes interfere with uricase-based measurement of circulating UA concentrations in blood samples. Due to the preservatory action of individual components, ACD tubes are commonly used in biobanking studies to gather and store large numbers of patient samples for reliable metabolite analysis. However, our results indicate that citric acid interferes with the peroxidase activity in the uricase method by scavenging hydrogen peroxide. Based upon our reasoning, it is also possible that citric acid would interfere with the measurements of other circulating metabolites that rely on peroxidase activity for measurement such as total cholesterol and triglyceride concentrations. Additionally, it is entirely possible that increased concentrations of antioxidants in blood may falsely lower measured UA concentrations. We suggest that clinicians and others using banked blood samples for research endeavors be highly aware of these effects on uricase-based UA measurements.
Although previous studies have demonstrated the variability of UA measurements based on sample type using mass spectrometry and other techniques [5–12], none have compared measurements using the more clinically-relevant uricase method. Taken together, our findings indicate that samples collected using ACD tubes (or tubes containing citric acid and/or sodium citrate) should not be used for the measurement UA concentration when using the uricase measurement method, as they provide unpredictably low values.
5. Conclusion
UA concentrations of blood samples collected using ACD tubes are falsely and unpredictably low when using the uricase-method of measurement. Our results demonstrate that citric acid interferes with UA measurement by stabilizing hydrogen peroxide that is produced as a uricase reaction intermediate—an intermediate vital to accurate UA measurement. Variable concentrations of citric acid may also be present in tubes utilizing sodium citrate as an anticoagulant, as citric acid is the conjugate acid of sodium citrate. Although we were unable to determine a mechanism by which dextrose interferes with measurement, our results show that blood samples collected in high concentrations of dextrose also skewed UA measurement. Consequently, we recommend that clinicians avoid using ACD and SC tubes to measure circulating UA concentrations in a clinical or biobank setting. Based on this study, we recommend using SST tubes for detection of UA in serum and lithium heparin tubes for detection of UA in plasma when using the lecuo dye detection method.
Highlights:
Plasma from Acid Citric Dextrose (ACD) tubes decreases measured Uric acid concentrations.
Citric acid in ACD tubes interferes with the uricase-based method of detection.
Scavenging of hydrogen peroxide by citric acid competes for the leuco dye substrate.
Clinicians should avoid tubes containing citric acid when measuring uric acid.
Acknowledgements
The authors thank the expertise of the Clinical Laboratory Technicians Elizabeth Schoenecker, Risa Urbauer, Flordeliza Faulkner, Nicole Norotsky at the Omaha Veteran’s Affairs Medical Center for their contributions to the results of this study.
Funding:
This research was supported by the University of Nebraska Medical Center Enhanced Medical Education Tract in Autoimmune Diseases. Dr. Mikuls is supported by grants from the National Institute of General Medical Sciences (U54GM115458); National Institute on Alcohol Abuse and Alcoholism (R25AA020818) and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
Abbreviations:
- UA
uric acid
- EDTA
ethylenediaminetetraacetic acid
- ACD
Acid Citrate Dextrose
- SST tube
Serum Separator Tube
- SC
Sodium Citrate
- LH
Lithium Heparin
- SSTua
Serum Separator Tube uric acid
- ACDua
Acid Citrate Dextrose uric acid
- VARA
Veteran’s Affairs Rheumatoid Arthritis
- PBS
phosphate buffered saline
- 4-APP
4-aminoantipyrine
- MADB
N,N-bis(4-sufobutyl)-3,5-dimethylaniline, disodium salt
- LHua
Lithium Heparin uric acid
- SCua
Sodium Citrate uric acid
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Novelty Statement
This manuscript provides evidence for a falsely low Uric Acid value using the uricase leuco dye measurement method when blood is collected in tubes containing citric acid or sodium citrate.
Declarations of Interest:
None
References
- [1].Cannella AC, Mikuls TR, Understanding treatments for gout, Am J Manag Care 11(15 Suppl) (2005) S451–8; quiz S465–8. [PubMed] [Google Scholar]
- [2].Martillo MA, Nazzal L, Crittenden DB, The crystallization of monosodium urate, Curr Rheumatol Rep 16(2) (2014) 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M, Comorbidities in patients with gout prior to and following diagnosis: case-control study, Ann Rheum Dis 75(1) (2016) 210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Roddy E, Choi HK, Epidemiology of gout, Rheum Dis Clin North Am 40(2) (2014) 155–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bowen RA, Remaley AT, Interferences from blood collection tube components on clinical chemistry assays, Biochem Med (Zagreb) 24(1) (2014) 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liu L, Aa J, Wang G, Yan B, Zhang Y, Wang X, Zhao C, Cao B, Shi J, Li M, Zheng T, Zheng Y, Hao G, Zhou F, Sun J, Wu Z, Differences in metabolite profile between blood plasma and serum, Anal Biochem 406(2) (2010) 105–12. [DOI] [PubMed] [Google Scholar]
- [7].Barri T, Dragsted LO, UPLC-ESI-QTOF/MS and multivariate data analysis for blood plasma and serum metabolomics: effect of experimental artefacts and anticoagulant, Anal Chim Acta 768 (2013) 118–28. [DOI] [PubMed] [Google Scholar]
- [8].Liao LT, Liao CC, Liu CC, Yang TY, Wang GC, Evaluation of an electrochemical biosensor for uric acid measurement in human whole blood samples, Clin Chim Acta 436 (2014) 72–7. [DOI] [PubMed] [Google Scholar]
- [9].Wedge DC, Allwood JW, Dunn W, Vaughan AA, Simpson K, Brown M, Priest L, Blackhall FH, Whetton AD, Dive C, Goodacre R, Is serum or plasma more appropriate for intersubject comparisons in metabolomic studies? An assessment in patients with small-cell lung cancer, Anal Chem 83(17) (2011) 6689–97. [DOI] [PubMed] [Google Scholar]
- [10].Lopez-Bascon MA, Priego-Capote F, Peralbo-Molina A, Calderon-Santiago M, Luque de Castro MD, Influence of the collection tube on metabolomic changes in serum and plasma, Talanta 150 (2016) 681–9. [DOI] [PubMed] [Google Scholar]
- [11].Breier M, Wahl S, Prehn C, Fugmann M, Ferrari U, Weise M, Banning F, Seissler J, Grallert H, Adamski J, Lechner A, Targeted metabolomics identifies reliable and stable metabolites in human serum and plasma samples, PLoS One 9(2) (2014) e89728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bowen RA, Hortin GL, Csako G, Otanez OH, Remaley AT, Impact of blood collection devices on clinical chemistry assays, Clin Biochem 43(1–2) (2010) 4–25. [DOI] [PubMed] [Google Scholar]
- [13].England BR, Sayles H, Michaud K, Caplan L, Davis LA, Cannon GW, Sauer BC, Solow EB, Reimold AM, Kerr GS, Schwab P, Baker JF, Mikuls TR, Cause-Specific Mortality in Male US Veterans With Rheumatoid Arthritis, Arthritis Care Res (Hoboken) 68(1) (2016) 36–45. [DOI] [PubMed] [Google Scholar]
- [14].Abdel-Salam OM, Youness ER, Mohammed NA, Morsy SM, Omara EA, Sleem AA, Citric acid effects on brain and liver oxidative stress in lipopolysaccharide-treated mice, J Med Food 17(5) (2014) 588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jabeen H, Saleemi S, Razzaq H, Yaqub A, Shakoor S, Qureshi R, Investigating the scavenging of reactive oxygen species by antioxidants via theoretical and experimental methods, J Photochem Photobiol B 180 (2018) 268–275. [DOI] [PubMed] [Google Scholar]
- [16].Karyotou K, Donaldson RP, Ascorbate peroxidase, a scavenger of hydrogen peroxide in glyoxysomal membranes, Arch Biochem Biophys 434(2) (2005) 248–57. [DOI] [PubMed] [Google Scholar]
- [17].Sroka Z, Cisowski W, Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids, Food Chem Toxicol 41(6) (2003) 753–8. [DOI] [PubMed] [Google Scholar]
- [18].Cook DG, Shaper AG, Thelle DS, Whitehead TP, Serum uric acid, serum glucose and diabetes: relationships in a population study, Postgrad Med J 62(733) (1986) 1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


