FIG 2.
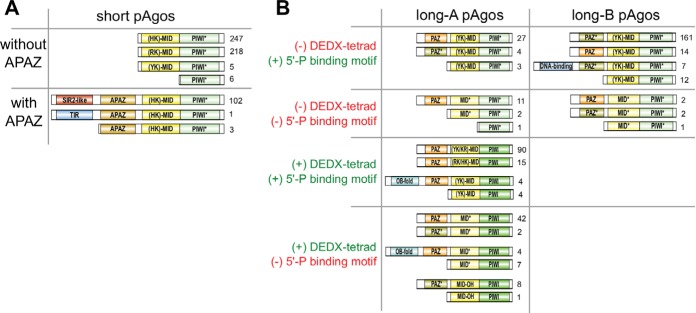
The diversity of domain architecture of pAgo proteins. Various types of domain organization of short (A) and long (B) pAgo proteins are schematically shown. The numbers on the right of each type are the frequency of its occurrence in the redundant set of proteins. Based on the presence of the DEDX tetrad in the PIWI domain and the putative 5′-P guide binding motif in the MID domain, all long pAgos can be separated into four types shown here. Green PIWI domains carry the active DEDX catalytic tetrad while turquoise PIWI* domains do not contain the canonical tetrad. Yellow MID domains contain different types of the 5′-end guide binding motif as indicated, while light-yellow MID* domains carry substitutions of the crucial amino acid residues in this motif. Orange PAZ domains have the full-sized pocket responsible for 3′-end guide binding, while light-brown PAZ* variants do not have the second subdomain. The OB-fold is the nucleic acid-binding domain SSF50249; the DNA-binding domains are Schlafen domain with AlbA_2 (PF04326) or lambda-repressor-like domain (SSF47413).