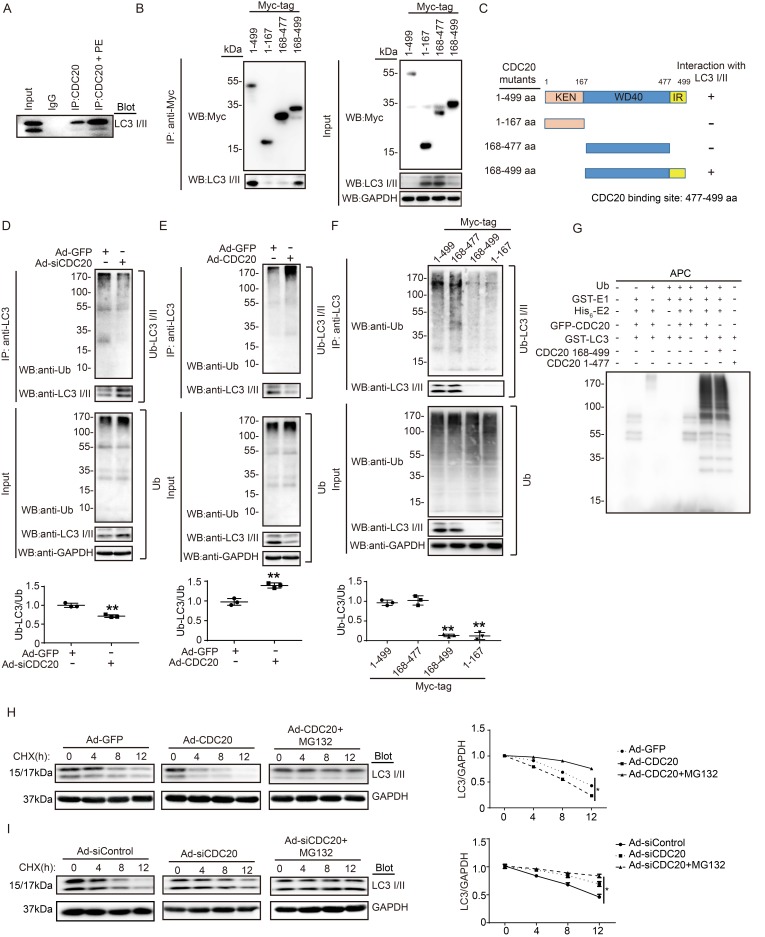
Figure 5.
CDC20 interacts with LC3 and directly promotes LC3 ubiquitination and degradation by the proteasome. (A) Western blot showing the expression of LC3 I/II by co-IP. (B) Western blot showing the expression of CDC20 mutant proteins (tagged with Myc) and LC3 I/II. (C) Schematic diagram depicting the CDC20 domain structure and summary of in vitro binding experiments. (D and E) Lysates harvested from NRCMs after infection with siRNA-control and siRNA-CDC20 or infection with Ad-GFP and Ad-CDC20 and pretreatment with MG132 for 6 hours were immunoprecipitated with anti-LC3. The ubiquitin-conjugated LC3 was detected by Western blotting with anti-ubiquitin (Ub) and anti-CDC20 antibodies (upper panel). Input showing the expression of the corresponding proteins in whole-cell lysates (lower panel). (F) Ubiquitin-conjugated LC3 was detected by Western blotting with anti-ubiquitin (Ub) (left). Input showing the expression of the corresponding proteins in whole-cell lysates (right). (G) CDC20 ubiquitinated LC3 in vitro. GFP-tagged CDC20 was tested for E3 ubiquitin ligase activity in the presence and absence of GST-E1, His6-E2, GFP-CDC20 (full-length 1-499 aa), GFP-CDC20 (168-499 aa) and GFP-CDC20 (1-476 aa). The protein blot was analyzed using an anti-ubiquitin antibody. (H and I) NRCMs were infected with adenovirus Ad-GFP and Ad-CDC20 or with siRNA-control and siRNA-CDC20 and then treated with cycloheximide (CHX, 10 μM) and/or MG132 (10 μM) and harvested at the indicated time points. Representative immunoblotting analyses of LC3 protein levels for each group.