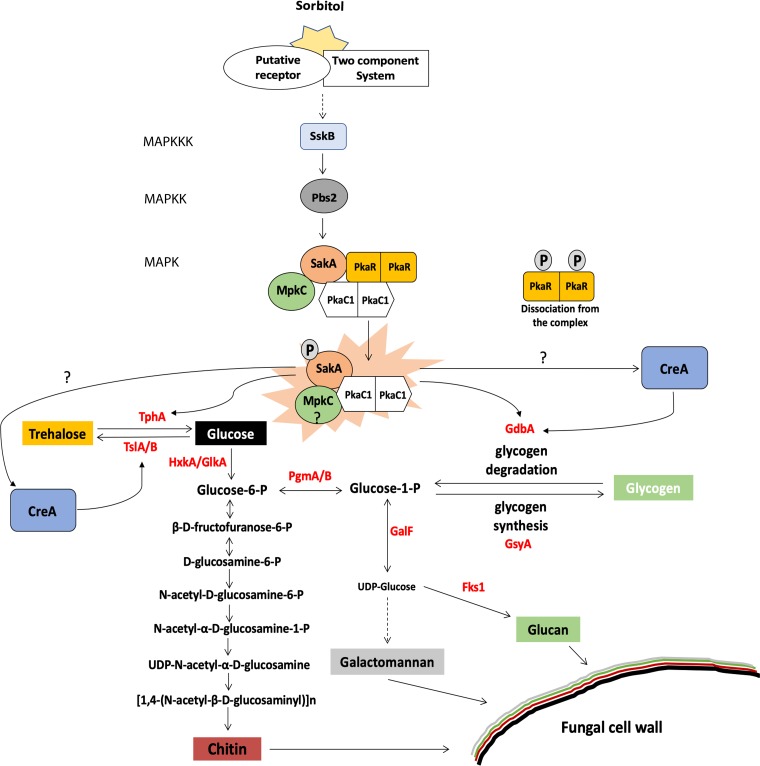
FIG 6.
The HOG and PKA pathways collaborate to regulate glycogen and trehalose metabolism for cell wall polysaccharide precursor biosynthesis during osmotic stress conditions. In the absence of osmotic stress, the PkaR and PkaC1 subunits form a protein complex with the HOG MAPK kinases SakA and MpkC. Upon the detection of osmotic stress, the HOG MAPK cascade is activated, signaling to Pbs2, which in turn phosphorylates SakA. The active SakA acts upon the PKA regulatory subunit, resulting in the dissociation of PkaR from the complex and the activation of PkaC1. The MpkC-SakA-PkaC1 complex can now control trehalose and glycogen metabolism to counteract the osmotic stress via two distinct pathways: (a) indirectly, through controlling the transcription factor of the carbon catabolite repression CreA that regulates the expression of genes encoding enzymes required for glycogen and trehalose utilization; and (b) directly, through controlling the activity of enzymes involved in glycogen and trehalose metabolism. The degradation of glycogen and trehalose results in the release of glucose, serving as a precursor for chitin, glucan, and galactomannan biosynthesis, through a variety of enzymatic steps. The chitin and galactomannan biosynthetic pathways were predicted based on homology with Saccharomyces cerevisiae using the BioCyc Pathway/Genome Database Collection website. Dashed arrows depict several steps in the pathway. TphA, trehalase phosphorylase; TslA/B, trehalose 6-phosphate synthase; HxkA, hexokinase; GlkA, glucokinase; PgmA/B, phosphoglucomutase; GalF, UTP-glucose-1-phosphate uridylyltransferase; Fks1, β-1,3-glucan synthase; GdbA, glycogen debranching enzyme; and GsyA, glycogen synthase.