Abstract
Centella asiatica (Umbelliferae) has been used for centuries in Indian ayurvedic medicine for the treatment of a wide number of health disorders. The aim of this study was to estimate and compare the concentration of bioactive compounds between wild and in vitro propagated C. asiatica plants. A marked decrease in the total phenolic compounds, flavonoids, and ascorbic acid was observed between in vitro propagated and wild type plants collected from wet land habitat. The radical scavenging activity of the wild type plant extracts also varied with the habitats. This study clearly indicates that environmental factors play a crucial role in the plant metabolic activity and medicinal activity.
Keywords: Centella asiatica, Callus, Free radicals, Medicinal plants
1. Introduction
Centella asiatica, a small herbaceous, annual plant in India, Sri Lanka, Malaysia, and other parts of Asia is a well known medicinal herb with a long history of therapeutic uses [6], [15]. The herb was used for wound healing, leprosy, skin diseases, microangiopathy, rheumatism, inflammation, syphilis, antibacterial, antifungal, and mental illness [23], [16]. The bioactive compounds present in C. asiatica include ursane-type triterpene saponin asiatic acid, asiaticoside, madecassic acid, madecassoside, etc., that are responsible for the wide therapeutic activity. However, a significant difference in active constituent content among samples from different locations has been reported [18]. It has been established that the climatic conditions, soil texture, and agronomic practices highly influence the bioactive constituent contents of C. asiatica [8], [20], [11], [24].
Rapid urbanization, degradation of plant habitats, ruthless collection of herbs, pollution, and other anthropogenic activities reduced the diversity of medicinal plants in the ecosystem [22]. The development of plant tissue culture technology holds great promise for conservation and enhancement of valuable medicinal plants [12]. In vitro propagation refers to true-to-type propagation of selected genotypes under laboratory conditions. Different explants such as single cells, protoplasts, pieces of leaves or roots can be used to generate a new plant on culture media with required nutrients [17]. Recent studies reported the in vitro propagation of a variety of medicinal plants [12]. As a result, efforts have been devoted to enhance the in vitro propagation of highly threatened valuable medicinal plants. However, the therapeutic efficiency of the propagated medicinal herbs was not reported. Hence, the present work aimed (i) to collect C. asiatica from different habitats, (ii) in vitro propagation of C. asiatica under laboratory conditions, and (iii) estimation and comparison of active constituents between micropropagated and wild plants from different habitats.
2. Materials and methods
2.1. Plant material and extraction
The young leaves of C. asiatica were collected from different natural habitats such as hill station, dry land, and wet lands, and carefully transported to the laboratory. Shade dried leaves were powdered (40 mesh size) and extracted (50–100 g) with methanol using Soxhlet extractor for 12–24 h [10]. The extract was filtered through Whatman No. 1 filter paper, concentrated under vacuum at 40 °C, freeze-dried at −80 °C, and stored at 4 °C until further use in the experiment.
2.2. In vitro culture–callus induction
Young leaves of C. asiatica collected from wet land habitat were used for the callus induction. The leaves were rinsed under running tap water for 30 min, wrapped up in 25% (v/v) clorox containing three drops of tween 20 solution for 10 min, and again rinsed several times with sterile distilled water until all traces of clorox were eliminated. Later, the leaves were cut into 5 mm × 5 mm in size and carefully transferred to the sterile Murashige and Skoog (MS) basal medium (pH 5.8) supplemented with 3% (w/v) sucrose, 0.8% agar, and plant growth regulators. Culture tubes were incubated at 24 ± 2 °C with 50 μmol m−2 s−1 irradiance provided by a cool fluorescent lamp with a photoperiod of 16 h [25]. Callus induced on MS medium was harvested, dried, and the dry weight of the total biomass was determined. One gram (dry weight) of callus was soaked in 10 ml of 80% methanol for 3 h and sonicated in an Ultrasonic Sonicator at 20 pulses for 20 min. The extract was centrifuged at 10,000 rpm for 10 min and the supernatant was freeze-dried and stored at 4 °C until further use in the experiment.
2.3. Induction of rooting
Excised shoots (1–2 cm) regenerated from the callus were cultured on rooting medium containing half strength basal MS semi-solid medium with different concentrations of Indole-3-butyric acid and 2% (w/v) sucrose for root initiation.
2.4. Determination of total phenolic contents
Total phenolic contents of the plant extract were estimated using Folin–Ciocalteu’s (FC) phenol reagent according to Kim et al. [14] with minor modifications. Briefly, 1 ml of standard solutions of gallic acid at different concentrations or diluted C. asiatica extracts was added to a 25 ml standard flask containing 9 ml of sterile distilled water. Later, 1 ml of FC phenol reagent was added to the flask, thoroughly mixed and incubated for another 5 min at room temperature. Ten milliliters of Na2CO3 solution (10% w/v) was added into the above mixture with constant stirring and immediately made up to volume (25 ml) with sterile distilled water. The reaction mixture was incubated at 23 °C for 1 h, and the absorbance was measured at 750 nm. Total phenolic contents in C. asiatica plants were expressed as mg gallic acid equivalents (GAE)/g dry weight [4]. Sterile distilled water was used as the negative control for this experiment. Experiments were repeated two times and mean values were considered.
2.5. Estimation of total flavonoids
An aliquot (0.5 ml) of the methanolic extract solution was mixed with 2 ml of sterile distilled water and 0.15 ml of 5% NaNO2 solution, and incubated for 5 min at 25 °C. After incubation, 0.15 ml of 10% AlCl3 solution was added to the above mixture and allowed to stand for additional 6 min at 25 °C. Later, 2 mL of 4% NaOH solution was added to the reaction mixture and was diluted to 5 ml volume with sterile distilled water. The reaction mixture was thoroughly mixed, incubated at 25 °C for 15 min, and the intensity of pink color was measured at 510 nm. Catechin was used to calculate the standard curve and the results were expressed as mg of catechin equivalents per g of methanolic extract [13], [2]. Sterile distilled water was used as the negative control for this experiment.
2.6. Analysis of total ascorbic acid
Total ascorbic acid concentration of the plant extract was estimated according to Klein and Perry (1982). Briefly, 1 ml of the filtrate was mixed with 9 ml of 2,6-dichloroindophenol (9 ml) and the absorbance was measured within 30 min at 515 nm. Total ascorbic acid was calculated on the basis of the calibration curve of l-ascorbic acid, and the results were expressed as mg of ascorbic acid per 100 g of dry weight. Sterile distilled water was used as the negative control for this experiment. Experiments were repeated two times and mean values were considered.
2.7. Analysis of free radical scavenging activity by DPPH
The scavenging activity for DPPH free radicals was measured according to Blios [3]. In brief, methanolic extract of the sample at various concentrations (5−25 μg/ml) was mixed with 5 ml of 0.1 mM methanolic DPPH solution and incubated for 20 min at 27 °C. After incubation, the absorbance of the reaction mixture was measured at 517 nm. Butylated hydroxyl toluene was used as standard. Sterile distilled water was used as the negative control for this experiment.
2.8. Thin layer chromatography (TLC) and DPPH staining
The methanolic extracts were loaded individually onto the baseline of silica gel and were dried for 1–2 h. The gel was stained with DPPH solution according to [7].
3. Results and discussion
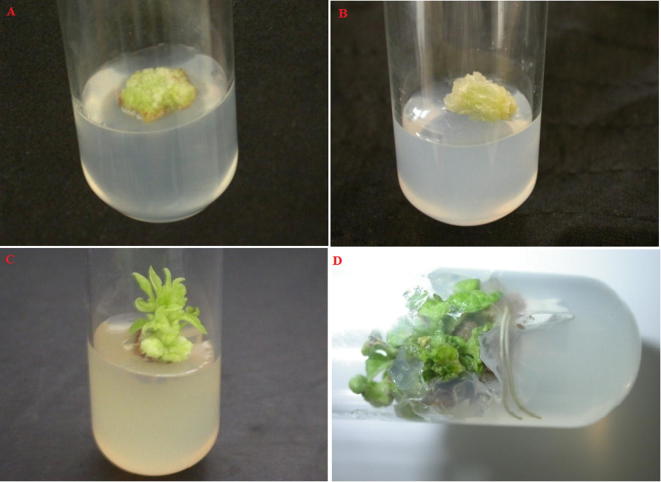
The study comprised an attempt to correlate the concentration and activity of bioactive compounds between wild and in vitro propagated C. asiatica plants. The plant collected from the wet land region was used for the callus induction because it is most commonly used in the medicinal practice. The callus induction was initiated after 3–4 weeks of incubation and both root and shoot were successfully regenerated from the propagated callus. The callus regeneration, shoot formation and rooting of C. asiatica plants are shown in Fig. 1.
Figure 1.
Stages of in vitro propagation of Centella asiatica (A) Callus formation, (B) Callus elongation, (C) Shoot regeneration and (D) Root regeneration.
Numerous studies reported that secondary metabolites present in the plant extracts play a vital role in therapeutic uses [18]. Thus, total phenolic compounds, flavonoids, and ascorbic acid content in C. asiatica collected under in vitro and in vivo conditions were analyzed and the results are shown in Table 1. The results clearly indicated that micropropagated plants have low concentrations of bioactive compounds compared to wet land plant. However, the concentration of bioactive compounds was high when compared with dry and hill station plants. Several reasons may explain the differences in concentration of bioactive compounds; genetic diversity, environmental conditions, and nutrients present in the soil/culture medium may alter the metabolic activity of plants. Alternatively, climatic conditions, bioavailability of nutrients, may alter the growth rate of explants, and thereby plant metabolic activity [5]. Moreover, it has been established that wet land region possesses optimum atmospheric and environmental conditions for enhanced plant growth and metabolic activity. The results are consistent with previous studies reporting the significant differences in secondary metabolite contents of C. asiatica growing in different habitats [9], [19], [18]. Even though the environmental conditions and nutrients were highly favorable for the callus induction, considerable difference in concentrations of bioactive compounds was observed between wet land and in vitro propagated plants. The differences in the light intensity, nutrient concentration, and rhizosphere microbial interactions may alter the growth rate of the plant cells and thereby metabolic activity [21], [1]. This phenomenon needs more study.
Table 1.
Phytochemical composition of C. asiatica collected from different habitats.
| S. No. | Phytochemicals | Habitats |
|||
|---|---|---|---|---|---|
| Dry land | Wet land | Hill station | In vitro | ||
| 1 | Total phenols (mg GAE/g DW) | 4 ± 1 | 10 ± 0.3 | 6 ± 0.3 | 6.2 ± 0.4 |
| 2 | Total flavonoids (mg/g) | 22 | 45 | 33 | 36 |
| 3 | Ascorbic acid (mg/g) | 18 | 35 | 24 | 27 |
Results are average of two replications ± standard error.
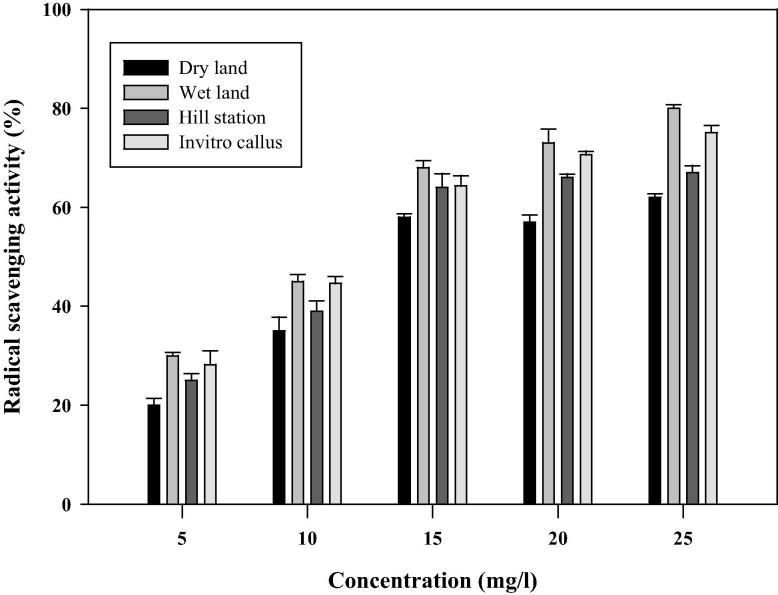
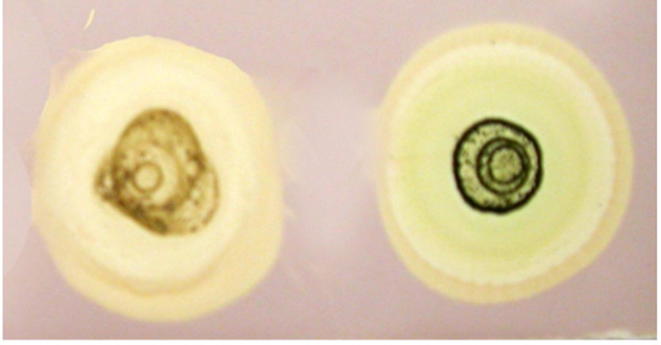
The radical scavenging capacity based on DPPH assay was determined and the results are shown in Fig. 2. A gradual increase in the scavenging effect on the DPPH radical was observed according to the concentrations (5–25 μg/ml) of the extract. The percentage of inhibition of DPPH radical was varied from 20% to 81% according to the concentration. The maximum activity was observed in the plants collected from wet land habitat (81%), followed by in vitro propagated callus (76%), hill station (67%), and dry lands (61%). The results were further confirmed by dot blot assay (Fig. 3). The appearance of the yellow color in the spots indirectly indicated the radical scavenging activity of the extracts. The increased radical scavenging activity in wet land plants was due to the high concentration of bioactive compounds. This was supported by the results of total phenolic compounds, total flavonoids, and ascorbic acid (Table 1). The high concentration of bioactive compounds was due to the favorable environmental and climatic conditions in the wet land regions [18].
Figure 2.
Radical scavenging activity of methanolic extract of Centella asiatica by the DPPH method. Error bar indicates the standard deviation of means.
Figure 3.
Thin layer chromatography of C. asiatica extract stained with a DPPH solution (color spots indicated the presence of radical scavenging activity).
4. Conclusions
The results of the study indicate that environmental conditions and other abiotic factors markedly affect the antioxidant activities of C. asiatica. A marked decrease in the concentration of metabolites was observed in in vitro propagated plants. Further work will address the optimization of environmental condition and other abiotic factors on metabolite yield and plant growth rate under in vitro conditions.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
Contributor Information
Muthusamy Govarthanan, Email: gova.muthu@gmail.com.
Thangasamy Selvankumar, Email: t_selvankumar@yahoo.com.
References
- 1.Barik D.P., Naik S.K., Mudgal A., Chand P.K. In Vitro Cell. Dev. Biol. Plant. 2007;43:144–148. [Google Scholar]
- 2.Barros L., Oliveira S., Carvalho A.M., Ferreira I.C.F.R. Ind. Crop. Prod. 2010;32:572–579. [Google Scholar]
- 3.Blios M.S. Nature. 1958;26:1199–1200. [Google Scholar]
- 4.Bouayed J., Piri K., Rammal H., Dicko A., Desor F., Younos C., Souliman R. Food Chem. 2007;104:364–368. [Google Scholar]
- 5.Bourgaud F., Gravot A., Milesi S., Gontier E. Plant Sci. 2001;161:839. [Google Scholar]
- 6.Brinkhaus B., Lindner M., Schuppan D., Hahn E.G. Phytomedicine. 2000;7:427–448. doi: 10.1016/s0944-7113(00)80065-3. [DOI] [PubMed] [Google Scholar]
- 7.Choi C.W., Kim S.C., Hwang S.S., Choi B.K., Ahn H.J., Lee M.Y., Park S.H., Kim S.K. Plant Sci. 2002;163:1161–1168. [Google Scholar]
- 8.Das A., Mallick R. Bot. Bull. Acad. Sin. 1991;32:1–8. [Google Scholar]
- 9.Djeridane A., Yousfi M., Nadjemi B., Boutassouna D., Stocker P., Vidal N. Food Chem. 2006;97:654–660. [Google Scholar]
- 10.Gafner F., Msonthi J.D., Hostettmann K. Helv. Chim. Acta. 1985;68:555–558. [Google Scholar]
- 11.Jamil S.S., Nizami Q., Salam M. Nat. Prod. Rad. 2007;6:158–170. [Google Scholar]
- 12.Jeyachandran R., Baskaran Xavier R., Cindrella L. Asian Pac. J. Trop. Biomed. 2012:S484–S487. [Google Scholar]
- 13.Jia Z., Tang M., Wu J. Food Chem. 1999;64:555–559. [Google Scholar]
- 14.Kim D.O., Jeong S.W., Lee C.Y. Food Chem. 2003;81:321–326. [Google Scholar]
- 15.Long H.S., Stander M.A., Van Wyk B.E. S. Afr. J. Bot. 2012;82:53–59. [Google Scholar]
- 16.Mamedov N. J. Mol. Cell. Biol. 2005;4:71–75. [Google Scholar]
- 17.Mohamed M.F., Read P.E., Coyne D.P. Plant Growth Regul. 1991;19:19–26. [Google Scholar]
- 18.Randriamampionona D., Diallo B., Rakotoniriana F., Rabemanantsoa C., Cheuk K., Corbisier A., Mahillon J., Ratsimamanga S., Jaziri M. Fitoterapia. 2007;78:482–489. doi: 10.1016/j.fitote.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Romani A., Vignolini P., Galardi C., Aroldi C., Vazzana C., Heimler D. J. Agric. Food. Chem. 2003;51:5301–5306. doi: 10.1021/jf0212136. [DOI] [PubMed] [Google Scholar]
- 20.Rouillard-Guellec F., Robin J.R., Rakoto-Ratsimamanga A., Ratsimamanga S., Rasaoanaivo P. Acta Bot. Gall. 1997;144:489–493. [Google Scholar]
- 21.Rout G.R. In Vitro Cell. Dev. Biol. Plant. 2005;41:516–519. [Google Scholar]
- 22.Shaik S., Singh N., Nicholas A. Plant Cell Tissue Organ Cult. 2011;105:439–446. [Google Scholar]
- 23.Shrestha P.M., Dhillion S.S. J. Ethnopharmacol. 2003;86:81–86. doi: 10.1016/s0378-8741(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 24.Siddiqui Y., Islam T.M., Naidu Y., Meon S. Sci. Hortic. (Amsterdam) 2011;130:289–295. [Google Scholar]
- 25.Singh J., Tiwari K.N. Ind. Crops Prod. 2012;35:224–229. [Google Scholar]