Abstract
The production of crop residues in India is estimated to be about 500–550 million tons annually. It is estimated that about 93 million tons of crop residues is burnt annually which is not only wastage of valuable biomass resources but pollution of the environment with the production of green house gases also. Among different low cost crop residues, black gram residue as the substrate produced maximal endoglucanase, FPase, and β-glucosidase activities from Aspergillus nidulans AKB-25 under solid-state fermentation. During optimisation of cultural parameters A. nidulans AKB-25 produced maximal endoglucanase (152.14 IU/gds), FPase (3.42 FPU/gds) and xylanase (2441.03 IU/gds) activities. The crude enzyme was found effective for the saccharification of pearl millet stover and bio-deinking of mixed office waste paper. The crude enzyme from A. nidulans AKB-25 produced maximum fermentable sugars of 546.91 mg/g from alkali-pretreated pearl millet stover by saccharification process at a dose of 15 FPU/g of substrate. Pulp brightness and deinking efficiency of mixed office waste paper improved by 4.6% and 25.01% respectively and mitigated dirt counts by 74.70% after bio-deinking. Physical strength properties like burst index, tensile index and double fold number were also improved during bio-deinking of mixed office waste paper.
Keywords: Black gram residue, Biomass-degrading enzymes, Aspergillus nidulans, Saccharification, Pearl millet stover, Bio-deinking
1. Introduction
India is primarily an agrarian country and one-third of the national income comes from agriculture. The production of crop residues in India is estimated to be about 500–550 million tons annually. The crop residues covers the whole range of biomass produced as by-products from growing and processing crops and it encompasses all agricultural wastes such as sugarcane bagasse, straw, stem, stalk, leaves, husk, shell, peel, pulp, stubble, etc. It is estimated that about 93 million tons of crop residues left in the field are burnt annually [1]. The crop residues are partially dried in the fields and a fraction is burnt to generate steam for the stripping; the rest is left in the fields where natural biodegradation takes place. The burning of crop residues is not only the wastage of valuable biomass resources which could be a source of carbon, bioactive compounds and energy but pollutes the environment by producing green house gases also. In India, various leguminous crops are grown for pulses, cattle forage and green manuring. The major pulse (leguminous) crops include Chickpea (Cicer arietinum), Pigeon pea (Cajanus cajan), Black gram (Vigna mungo) and Green gram (Vigna radiata). India is the world’s largest producer of pulses with its total pulse production contributing a quarter of world’s total production. Black gram is one of the pulses, mostly produced in Asian countries due to the tropical climate and soil type. The annual production of black gram is 1.40 million tons grown over an area of 3.10 million hectares which produces a huge amount of lignocellulosic residue [2], [3]. Black gram residue consists of cellulose (26.8 ± 2.3%), hemicellulose (32.48 ± 3.0%), lignin (23.14 ± 2.1%), crude protein (16 ± 0.8%), and ash content (5.1 ± 1.2%) [4].
Crop residues can serve as potential substrates for the production of various value added products like bioethanol, enzymes, organic acids, bio-surfactants, biogas, biohydrogen, and bio-fertilizers [5]. Crop residues are the inexpensive sources for the production of cellulases, hemicellulases and lignolytic enzymes. The hydrolysis of lignocellulosic biomass into fermentable sugars is the main factor for the high cost of ethanol production; the step of cellulase production accounts for 40% of total cost during ethanol production from cellulosic biomass [6]. Therefore, new microbial strains with higher production of cellulases and xylanases are required to reduce the cost of enzymes used in hydrolysis of lignocellulosic biomass [7]. Another strategy for cost reduction may be the use of low cost substrates for enzyme production and determination of optimum parameters of fermentation for enzyme production. Crop residues are very inexpensive materials for industrial production of enzymes. Solid state fermentation (SSF) can further reduce the cellulase and xylanase prices, because solid state fermentation results in more concentrated enzyme which is suitable for bioconversion of lignocellulosic biomass [8]. Cellulases and xylanases are very useful in many industries, such as textile, paper, bio-energy, animal feed, food and detergent. Cellulases and xylanases are predominantly used for enzymatic deinking and bio-bleaching in pulp and paper industry [9], [10], [11], [12].
Biomass-degrading enzymes are produced by many microorganisms, like fungi, bacteria and actinomycetes. However, fungi are the major sources of cellulolytic and hemicellulolytic enzymes due to higher yield of enzyme production [12]. Among fungi Trichoderma, Aspergilli, Penicillum, are the main genera which are used for the production of cellulases and xylanases. Trichoderma reesei is the most efficient producer of cellulases but it is deficient in β-glucosidase activity, leads to build up of cellobiose which causes the end product inhibition during cellulase production and hydrolysis of lignocellulosic biomass. Aspergillus species are effective in production of high level of β-glucosidase enzyme [12], [13], [14].
The present study aims at optimising various cultural conditions for the maximum production of endoglucanase, FPase, β-glucosidase and xylanase from Aspergillus nidulans AKB 25 using various by-products of crops residues under SSF. The applications of crude enzyme were observed (a) to produce fermentable sugars from pearl millet residue by saccharification process and (b) to deink mixed office waste paper by bio-deinking process. According to the literature survey, this is the first report to produce crude enzymes from A. nidulans using black gram residue as the substrate under SSF.
2. Methods
2.1. Isolation and identification of microorganism
The fungal isolate AKB-25 was isolated from the soil samples collected from Jaipur, Rajasthan, India by using carboxymethyl cellulose containing media. The fungal isolate was maintained over potato dextrose agar slants at 4 °C. Molecular phylogenetic and morphological studies were carried out for identification of fungal strain AKB-25. Based on morphological characteristics and phylogenetic analysis of ITS1-5.8S-ITS2 gene sequences, fungal isolate AKB-25 was identified as A. nidulans and submitted to NFCCI, Agharkar Research Institute, Pune (India) with accession number NFCCI 2977. The ITS sequences of A. nidulans AKB-25 were submitted to GenBank with accession numbers KP734017.
2.2. Crop residues
Different crop residues such as sugarcane bagasse, black gram residue, corn stover, pearl millet stover, rice straw, rice husk, sugarcane tops, sun hemp residue, wheat bran, wheat straw were collected locally. The mature plants of black gram were harvested from the surface of soil without roots and seeds were separated from the plants. The remaining part except root and seeds was taken as ‘black gram residue’ for enzyme production. All the crop residues were washed, dried and chopped into 1–2 cm pieces with fodder cutter. Chopped pieces were ground into smaller particles in a Wiley mill. Particle size range 250–1400 μm was used for enzyme production under SSF without any pretreatment. Pearl millet stover was pretreated with 3% NaOH at 121 °C for 20 min and a solid to liquid ratio of 1:8 was maintained. Alkali pretreated pearl millet stover was repeatedly washed with tap water until neutral pH achieved and was used for hydrolysis studies without drying.
2.3. Enzyme production and extraction
Enzyme production was carried out under SSF using sugarcane bagasse, black gram residue, corn stover, pearl millet stover, rice straw, rice husk sugarcane tops, sun hemp residue, wheat bran, and wheat straw as carbon sources. 5 g of each of the substrates was moistened with Mandel Weber medium (77.5% initial moisture content) having the following composition as g/l: 1.4 (NH4)2SO4, 2.0 KH2PO4, 0.3 CaCl2, 0.3 MgSO4.7H2O, 0.1 Tween-80 and trace elements: 0.005 FeSO4·7H2O, 0.0016 MnSO4·7H2O, 0.0014 ZnSO4·7H2O, 0.002 CoCl2·6H2O. The initial pH of Mandel Weber medium for enzyme production was adjusted to 5.5. The pH of Mendel’s mineral medium was adjusted with 1 N HCl or 1 N NaOH. 250 ml Erlenmeyer flasks containing 5 g of substrate were autoclaved at 121 °C for 30 min for sterilisation. Inoculum was prepared by harvesting spores from 7 days old wheat bran agar slants (3% wheat bran) by using 5 ml sterile distilled water containing 0.1% (w/v) Tween-80. Spore suspension was collected aseptically. Flasks were inoculated with 106 spores/gds and incubated at 30 °C for 6 days and left unperturbed. Initially, incubation time was kept as 6 days and after optimisation of incubation time flasks were incubated for 4 days. Enzyme was extracted by addition 50 ml of distilled water and flasks were shaken in an incubator shaker at 150 rpm and 30 °C for 60 min. The obtained supernatant was analysed for Endoglucanase, FPase, β-glucosidase and xylanase activities.
2.4. Enzyme assays
Cellulases are a multi-component enzyme system which consists of three major groups of enzymes: endo-β-1, 4-glucanases, exo-β-1, 4-glucanase and β-glucosidases. Total cellulase activity represents the collective measurement of endoglucanase, exoglucanase and β-glucosidases. Total cellulase activity was determined as filter paper (FPase) activity by using Whatman No. 1 filter paper as substrate. Endoglucanases randomly cleave β-1, 4-glycosidic linkages on the amorphous part of cellulose away from chain ends and was determined by using carboxymethyl cellulose (CMC) as substrate. Exoglucanase produces cellobiose by attacking cellulose from reducing and non-reducing chain ends and β-glucosidase converts cellobiose into glucose [8]. FPase and endoglucanase activities were determined by standard methods which are recommended by International Union of Pure and Applied Chemistry (IUPAC) [15].
For determination of FPase activity, 0.5 ml of appropriately diluted enzyme was thoroughly mixed with 1 ml of 50 mM citrate buffer (pH 5.5). After pre-incubation at 50 °C, a strip of Whatman No. 1 filter paper of dimensions 1 × 6 cm (50 mg) was kept in the mixture. The reaction mixture was incubated in a water bath at 50 °C for 60 min. 3 ml of DNS reagent was added to the reaction mixture and boiled in oil for 5.0 min in a vigorously boiling water bath containing sufficient water. A 0.2 mL of reaction mixture was diluted by adding 2.5 mL of distilled water, and the absorbance was measured at 540 nm. One filter paper unit was defined as the amount of enzyme that liberates 1 μmol glucose per ml per minute under assay conditions [15].
For endoglucanase activity, reaction mixture of 0.5 ml of 2% (w/v) carboxymethyl cellulose of medium viscosity (Sigma Chemical Co. St Louis, MO, USA), prepared in 50 mM citrate buffer (pH 5.5) and 0.5 ml of appropriately diluted crude enzyme was incubated at 50 °C for 30 min. The subsequent process for endoglucanase activity was the same as mentioned in FPase assay [15]. One unit of endoglucanase activity was defined as th eamount of enzyme that releases 1 μmole of glucose under specified conditions.
β-Glucosidase activity was assayed by using 5 mM p-nitrophenyl-β-d-glucopyranoside, prepared in 50 mM citrate buffer (pH 5.5) according to Wood and Bhat [16]. β-Glucosidase activity was determined by incubating 0.5 ml of appropriately diluted enzyme and 0.5 ml of p-nitrophenyl-β-d-glucopyranoside at 50 °C for 30 min. After incubation, 4.0 ml of glycine buffer was added to stop the reaction and absorbance was measured at 430 nm by UV–Visible spectrophotometer. One unit of β-glucosidase activity is defined as amount of enzyme that releases 1 μmole of p-nitro phenol per min per millilitre under assay conditions.
Xylanase activity was determined by estimating the reducing sugars released by 1% (w/v) of beechwood xylan (Sigma Chemical Co. St Louis, MO, USA), prepared in 50 mM citrate buffer (pH 5.5) according to Bailey method [17]. Xylanase activity was determined by incubating 0.5 ml of appropriately diluted enzyme and 0.5 ml of beechwood xylan at 50 °C for 15 min. 2 ml of DNS reagent was added to reaction mixture and boiled for 5.0 min in a vigorously boiling water bath containing sufficient water and absorbance was measured at 540 nm. One unit of xylanase activity is defined as amount of enzyme that releases 1 μmole of xylose per min per millilitre under reaction conditions. Enzyme activities were expressed as activity units per mass of initial dry solid substrates.
2.5. Optimisation of parameters for enzyme production
Optimisation of enzyme production parameters was carried by one factor at a time (OFAT) approach [8], [18]. To screen the suitable carbon source for enzyme production various crop residues were tested. The effect of different parameters such as incubation time (1–7 days), temperature (26–42 °C), initial pH (3–10), and moisture content (55–85%) was investigated. The enzyme production by the fungus was investigated by supplementing the different simple and complex nitrogen sources at four different concentrations. Then different surfactants were evaluated for their role in enzyme production.
2.6. Enzymatic hydrolysis of pearl millet stover
Enzymatic hydrolysis of alkali pretreated pearl millet stover was conducted at 2% (w/v) insoluble solid substrate in 50 ml buffer solutions at pH 5.0 using crude enzyme containing cellulase, β-glucosidase and xylanase on the basis of 5–20 FPU/g of dry pearl millet stover. The hydrolysis experiments were conducted in an incubator shaker (New Brunswick Scientific, Innova® 43, USA) at 50 °C and 120 rpm for 96 h in the presence of 0.01% sodium azide. Hydrolysate samples were withdrawn at regular intervals of 12 h and centrifuged at 9000 rpm for 15 min to remove solids.
2.7. Bio-deinking of mixed office waste (MOW)
MOW paper was collected from Century Paper Mills, Lalkuan, District Nainital (Uttarakhand) India. MOW paper was torn manually into small pieces of size 1.5–2.5 cm2 and soaked in water at 30 °C for 60 min. Pulping was carried out in a hydrapulper (Universal Engineering Corporation, Saharanpur, India) at 600 rpm for a pulping time of 20 min. After hydrapulping, enzymatic treatment was given at different enzyme doses that varied from 0 to 5.0 IU endoglucanase/g of pulp (on oven dry basis) at 50 °C for 60 min. Enzyme doses were diluted with normal water according to pulp consistency maintained before enzyme treatment stage for good mixing of enzymes with the pulp. Further, enzymatically treated pulp was subjected to ink flotation and washing stages respectively to remove detached ink particles from the pulp. The deinked pulp pads were prepared on büchner funnel (Tappi T 218 sp-02 “Forming handsheets for reflectance testing of pulp, Büchner funnel procedure”) and tested for pulp brightness (Tappi T 452 om-08 “Brightness of pulp, paper, and paperboard, directional reflectance at 457 nm”). The deinked pulp samples were evaluated for pulp yield. Laboratory handsheets of 60 g/m2 were prepared (Tappi T 205 sp-02 “Forming handsheets for physical tests of pulp”) using a British handsheet former. The handsheets were conditioned at 27 ± 2 °C and relative humidity of 65 ± 2% for 24 h and evaluated for burst index (Tappi T-403 om-02 “Bursting strength of paper”), tensile index (Tappi T-404 wd-03 “Tensile breaking strength and elongation of paper and paperboard”), double fold (Tappi T-423 cm-98 “Folding endurance of paper”) and tear index (Tappi T 414 om-04 “Internal tearing resistance of paper”) [19]. Reducing sugars in pulp filtrate were determined by 3, 5-Dinitrosalicylic acid (DNS) method [20].
3. Results and discussion
3.1. Production of enzyme under SSF
Initially, A. nidulans AKB-25 produced endoglucanase (26.72 IU/gds), FPase (1.13 FPU/gds), β-glucosidase (30.94 IU/gds) and xylanase (1349.43 IU/gds) using wheat bran as substrate at 30 °C, pH 5.5, moisture content 77.5% after 6 days of incubation under SSF conditions. After that, screening of crop residues and optimisation of cultural parameters were carried out to cause maximum enzyme production.
3.2. Screening of crop residues for enzyme production by Aspergillus nidulans AKB-25
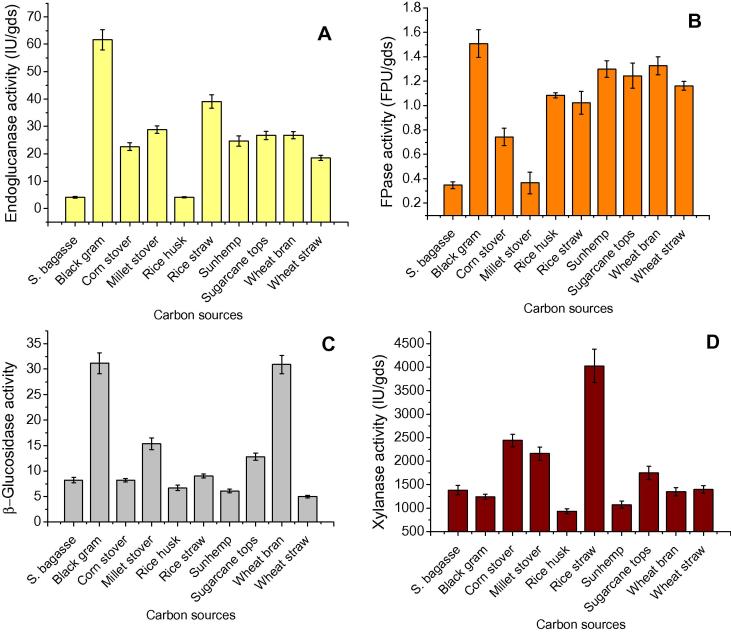
The effect of different carbon sources (sugarcane bagasse, black gram residue, corn stover, pearl millet stover, rice straw, rice husk, sugarcane tops, sun hemp residue, wheat bran and wheat straw) on enzyme production was evaluated based on endoglucanase, FPase and β-glucosidase activities. Maximum endoglucanase production was found on black gram residue (61.68 IU/gds), followed by rice straw (39.06 IU/gds) and pearl millet stover (28.78 IU/gds) (Fig. 1). Black gram residue was found to produce maximum FPase (1.50 IU/gds) followed by wheat bran (1.32 IU/gds), sun hemp residue (1.29 IU/gds) and sugarcane tops (1.24 IU/gds). Maximum β-glucosidase activities i.e. 31.15 and 30.94 IU/gds were observed on black gram residue and wheat bran respectively. Among crop residues tested, rice straw showed maximum xylanase activity (4024.59 IU/gds) while minimum xylanase activity was observed with black gram residue (1243.53 IU/gds) and wheat bran (1339.43 IU/gds). Finally, black gram residue was observed to produce maximum endoglucanase, FPase, and β-glucosidase activities and chosen as the carbon source for further experiments to optimise the other cultural conditions. The selection of carbon source as the substrate for biomass degrading enzymes production was considered based on the cost, abundance and physico-chemical characteristics of substrates as described by Delabona et al. [21]. Enzyme production is dependent on the nature of carbon source, favourable degradability, bare chemical composition, physical associations, accessibility of substrate, and presence of some nutrients [22]. Jabasingh and Nachiyar [23] observed maximum endoglucanase activity (28.84 IU/gds) with A. nidulans MTCC344 using sugarcane bagasse as the substrate. Vitcosque et al. [24] showed that Aspergillus niger produced maximum FPase (0.55 IU/gds), endoglucanase (35.1 IU/gds), xylanase (47.7 IU/gds) activities at 84% moisture content using soybean meal as the solid substrate. Pirota et al. [25] found maximum FPase (0.25 IU/gds), endoglucanase (113.4 IU/gds) and β-glucosidase (2.0 IU/gds) and xylanase (507.9 IU/gds) activities by Aspergillus oryzae P27C3A using wheat bran as the carbon source. Dhillon et al. [26] reported maximum β-glucosidase 21.69 IU/gds and 13.58 IU/gds by A. niger and T. reesei respectively on wheat bran medium after 4 days of incubation.
Figure 1.
Endoglucanase (A), FPase (B), β-glucosidase (C) and xylanase (D) production by Aspergillus nidulans AKB-25 at temperature 30 °C, pH 5.5, moisture content 77.5% after 6 days of incubation period using different substrates (crop residues) under SSF. Results are presented as mean ± standard deviation.
3.3. Effect of cultural conditions on enzyme production
3.3.1. Incubation time
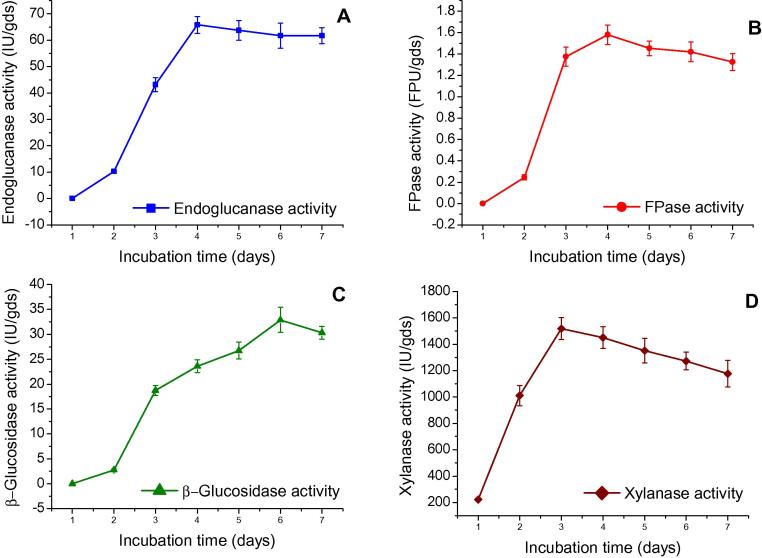
Fig. 2 shows that A. nidulans AKB-25 produced maximum endoglucanase (65.79 IU/gds) and FPase (1.58 FPU/gds) activities on the 4th day of incubation and after that it did not show any increment in endoglucanase and FPase activities. It may be due to depletion of nutrients and increase in toxic waste which results a decrease in growth and inactivation of secretary machinery of enzymes [27], [28]. Maximum xylanase activity (1518.85 IU/gds) was obtained on the 3rd day of incubation and decreased thereafter. Xylanases have an important role in the degradation of native lignocellulosic materials and their synthesis occurs in very early stage and before the production of cellulase [29]. Vitcosque et al. [24] also observed similar findings as they found maximum xylanase activity after 48 h while maximum FPase and endoglucanase activities were obtained after 96 h by A. niger. Production of β- glucosidase by A. nidulans AKB-25 increased on the 5th day of incubation and attained to its maximum on the 6th day of incubation. The present findings are in agreement with the findings of Gautam et al. [28] who reported maximum FPase and endoglucanase production on the 4th day while maximum glucosidase activity on 3-5th day of incubation.
Figure 2.
Effect of incubation period on enzyme production by Aspergillus nidulans AKB-25: Endoglucanase (A), FPase (B), β-glucosidase (C) and xylanase (D) production at temperature 30 °C, pH 5.5, moisture content 77.5% and incubation time varied from 1 to 7 days. Results are presented as mean ± standard deviation.
3.3.2. Temperature
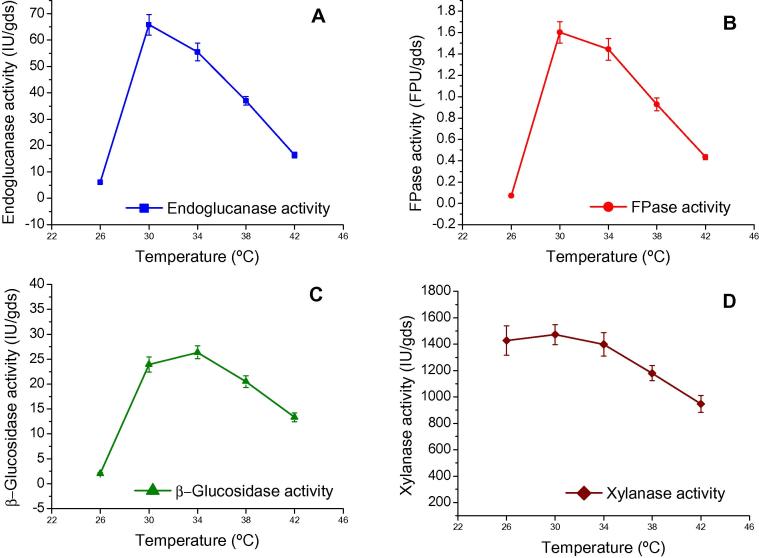
Temperature plays an important role for the production of enzymes by microorganisms; hence, it is necessary to optimise temperature for cellulolytic and hemicellulolytic enzymes. The maximum endoglucanase (65.79 IU/gds) and FPase activities (1.60 FPU/gds) were observed at 30 °C and decreased thereafter. Maximum β-glucosidase activity (26.36 IU/gds) was found at 34 °C (Fig. 3). Enzyme is a secondary metabolite produced during exponential growth phase; the incubation at higher temperature could lead to a poor growth and thus, a reduction in enzyme yield [30], [31]. It is interesting to note that maximum xylanase activities (1472.17 IU/gds and1428.46 IU/gds) were observed at 30 and 26 °C respectively. The high titre of xylanase production compared to cellulase titre at lower temperature may be due to higher solubilisation of hemicelluloses than cellulose [32], [33]. The results obtained were in agreement with Dhillon et al. [26] who observed the highest endoglucanase (48.22 IU/gds), β-glucosidase (21.69 IU/gds) and xylanase (2604.06 IU/gds) activities under SSF at 30 °C using wheat bran as the substrate with A. niger. Deswal et al. [18] also reported an increase in cellulase production on wheat bran as the substrate by Fomitopsis sp. up to 30 °C and thereafter production of enzyme decreased.
Figure 3.
Effect of temperature on enzyme production by Aspergillus nidulans AKB-25: endoglucanase (A), FPase (B), β-glucosidase (C), and xylanase (D) production at incubation time 4th day, pH 5.5, moisture content 77.5% and temperature varied from 26 to 42 °C with an interval of 4 °C. Results are presented as mean ± standard deviation.
3.3.3. Initial pH
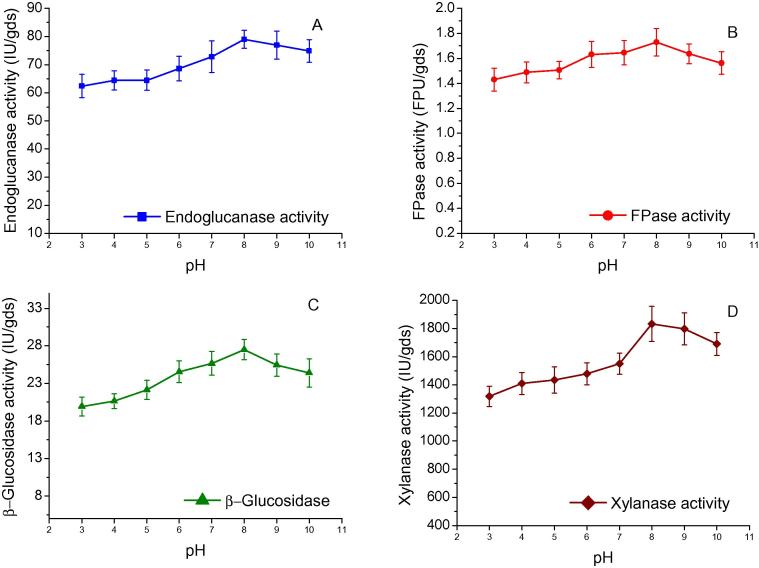
pH is an important environmental factor which affects the enzyme production under SSF. The effect of initial pH on enzyme production was investigated between pH 3.0 and 10.0 with a gap of one pH unit. The maximum endoglucanase (79.00 IU/gds), FPase (1.72 FPU/gds), β-glucosidase (27.50 IU/gds) and xylanase (1832.25 IU/gds) activities were observed at initial pH of 8.0 (Fig. 4). Maintenance of alkaline pH during the growth of A. nidulans AKB-25 showed the tolerance of fungus towards alkaline range of pH. Finally, the pH of all the crude enzymes extracted at different pH from A. nidulans AKB-25 are found in the alkaline range of 7.89–8.33. It shows that the pH of the crude enzyme under SSF conditions is difficult to control and substrate used in SSF condition usually have buffering effect due to their complex chemical composition [34].
Figure 4.
Effect of pH on enzyme production by Aspergillus nidulans AKB-25: Endoglucanase (A), FPase (B), β-glucosidase (C), and xylanase (D) production at incubation time 4th day, temperature 30 °C, moisture content 77.5% and pH varied from 3.0 to 10.0 with an interval of 1 unit. Results are presented as mean ± standard deviation.
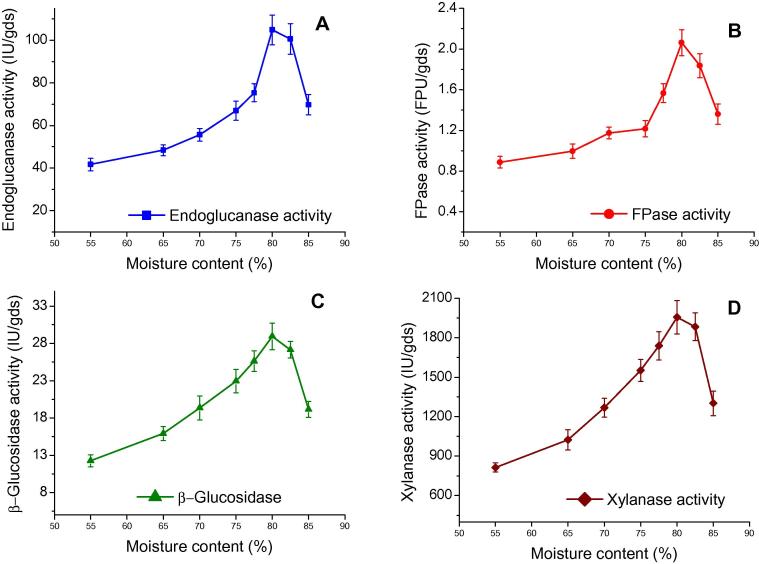
3.3.4. Initial moisture content
Moisture content is a crucial factor for enzyme production under SSF system. Therefore, it is necessary to find out the optimum moisture level for obtaining maximal fungal growth and secretion of different metabolites such as enzymes. An increase in initial moisture content from 55.0% to 80.0% greatly enhanced the endoglucanase, FPase, β-glucosidase, and xylanase production. Maximum endoglucanase (104.85 IU/gds), FPase (2.06 FPU/gds), β-glucosidase (28.94 IU/gds), and xylanase (1955.24 IU/gds) activities were observed at 80% moisture content and thereafter resulted into lower enzyme production titre for endoglucanase, FPase, β-glucosidase, and xylanase (Fig. 5). The moisture content of 55–90% under SSF for optimum enzyme production has been described in literature using different microorganisms and substrates. A lower moisture level may cause reduction in lignin solubility and swelling capacity of substrate [35]. Higher moisture content results swelling of substrate, thereby facilitating better utilisation of substrate by the microorganism [36]. If the moisture level is very high, void spaces in substrate are filled up with water which limits the oxygen supply and mitigates the substrate porosity and bonds the particles together due to gummy structure. However, optimum moisture level for enzyme production depends on both microorganism as well as nature of solid substrate used [24], [37]. Pirota et al. [25] observed maximum FPase (0.13 IU/gds), endoglucanase (115.9 IU/gds), β-glucosidase (2.70 IU/gds) and xylanase activities (1658.10 IU/gds) at 80% moisture contents by A oryzae P6B2 in SSF.
Figure 5.
Effect of moisture content on enzyme production by Aspergillus nidulans AKB-25: endoglucanase (A), FPase (B), β-glucosidase (C), and xylanase (D) production at incubation time 4th day, temperature 30 °C, pH 8.0 and moisture content varied from 55% to 85%. Results are presented as mean ± standard deviation.
3.3.5. Nitrogen sources
The nitrogen source used in production medium is one of the major factors affecting level of enzyme production. Therefore, the effect of various nitrogen sources at four different dosages was examined. Among different complex nitrogen sources, a dose of 0.8% (w/v) peptone was found most effective for the production of endoglucanase (127.47 IU/gds) and FPase (2.48 IU/gds) while maximum xylanase production (2139.98 IU/gds) was observed with 1.2% (w/v) yeast extract (Table 1). Urea and ammonia salts such as (NH4)2SO4, NH4Cl, (NH4)2PO4, and NH4NO3 (all taken as available N) were found effective for endoglucanase, FPase, and xylanase production by A. nidulans AKB-25. Among various simple nitrogen sources tested, 0.12% (NH4)2SO4 (as available N) gave maximum, endoglucanase (133.64 IU/gds), FPase (2.94 FPU/gds) and xylanase (2305.45 IU/gds) activities under SSF condition. The effect of nitrogen source on enzyme production depends upon nature fungal strains being employed. In various investigations, ammonium salts have been reported for higher cellulase production [8], [38]. In the present study, simple nitrogen sources were found to be more effective in comparison to complex nitrogen sources. Kalogeris et al. [39] reported enhanced enzyme activities of crude enzyme from Thermoascus aurantiacus with inorganic nitrogen sources compared to organic nitrogen sources under SSF condition. Similar findings were reported by Vyas et al. [40] who observed (NH4)2SO4 as the best nitrogen source for cellulase production by Aspergillus terreus under SSF on groundnut shell as the substrate.
Table 1.
Effect of nitrogen sources on enzyme production by Aspergillus nidulans AKB-25.
| Enzyme activities⁎ | Level of nitrogen sources on the basis of percentage of nitrogen |
||||
|---|---|---|---|---|---|
| 0.04% | 0.08% | 0.12% | 0.16% | ||
| Simple nitrogen sources | |||||
| NH4Cl | Endoglucanase | 106.91 ± 6.20 | 115.13 ± 5.71 | 119.24 ± 6.80 | 98.68 ± 5.37 |
| FPase | 2.42 ± 0.11 | 2.58 ± 0.15 | 2.63 ± 0.19 | 1.99 ± 0.12 | |
| Xylanase | 1813.98 ± 107.20 | 1886.83 ± 93.39 | 2117.75 ± 112.66 | 1895.48 ± 76.95 | |
| NH4NO3 | Endoglucanase | 108.96 ± 7.01 | 123.36 ± 8.11 | 111.02 ± 7.12 | 92.52 ± 6.16 |
| FPase | 1.96 ± 0.10 | 2.37 ± 0.15 | 2.03 ± 0.13 | 1.68 ± 0.09 | |
| Xylanase | 1613.93 ± 99.25 | 1767.05 ± 71.74 | 1613.93 ± 55.51 | 1601.59 ± 93.21 | |
| (NH4)2PO4 | Endoglucanase | 100.74 ± 6.32 | 119.24 ± 8.33 | 111.02 ± 8.62 | 96.63 ± 5.66 |
| FPase | 2.18 ± 0.15 | 2.44 ± 0.16 | 2.20 ± 0.10 | 1.84 ± 0.11 | |
| Xylanase | 1646.04 ± 97.28 | 2014.02 ± 114.59 | 2042.05 ± 140.28 | 1848.55 ± 104.44 | |
| (NH4)2SO4 | Endoglucanase | 117.19 ± 7.19 | 127.47 ± 7.99 | 133.64 ± 6.82 | 129.52 ± 7.47 |
| FPase | 2.23 ± 0.12 | 2.43 ± 0.14 | 2.94 ± 0.16 | 2.61 ± 0.12 | |
| Xylanase | 1843.62 ± 81.85 | 2135.04 ± 121.48 | 2305.45 ± 156.54 | 2053.54 ± 132.45 | |
| NaNO3 | Endoglucanase | 82.24 ± 4.30 | 88.40 ± 5.62 | 90.46 ± 5.18 | 84.29 ± 5.74 |
| FPase | 1.59 ± 0.10 | 1.88 ± 0.12 | 1.80 ± 0.11 | 1.66 ± 0.11 | |
| Xylanase | 1343.50 ± 71.47 | 1479.34 ± 80.32 | 1543.55 ± 94.92 | 1395.37 ± 106.04 | |
| Urea | Endoglucanase | 125.4 ± 6.19 | 123.36 ± 5.51 | 119.24 ± 6.33 | 96.63 ± 5.41 |
| FPase | 1.92 ± 0.10 | 2.52 ± 0.16 | 2.48 ± 0.13 | 2.20 ± 0.10 | |
| Xylanase | 1471.93 ± 82.42 | 1893.01 ± 125.88 | 1953.52 ± 160.97 | 1452.17 ± 106.44 | |
| Level of nitrogen sources on the basis of percentage (w/v) | |||||
| 0.4% (w/v) | 0.8% (w/v) | 1.2% (w/v) | 1.6% (w/v) | ||
| Complex nitrogen sources | |||||
| Beef | Endoglucanase | 108.96 ± 5.68 | 117.19 ± 7.41 | 127.47 ± 8.27 | 121.30 ± 7.01 |
| FPase | 1.75 ± 0.12 | 2.15 ± 0.11 | 2.15 ± 0.15 | 2.04 ± 0.11 | |
| Xylanase | 1343.50 ± 78.72 | 1436.12 ± 71.51 | 1634.93 ± 116.57 | 1513.91 ± 74.03 | |
| Malt | Endoglucanase | 74.01 ± 4.38 | 76.07 ± 4.15 | 71.96 ± 3.66 | 69.90 ± 4.37 |
| FPase | 1.11 ± 0.09 | 1.24 ± 0.07 | 1.19 ± 0.06 | 1.14 ± 0.07 | |
| Xylanase | 1216.31 ± 64.95 | 1134.82 ± 69.33 | 1070.60 ± 53.42 | 979.22 ± 47.78 | |
| Peptone | Endoglucanase | 88.35 ± 6.06 | 127.47 ± 8.60 | 117.13 ± 8.60 | 115.13 ± 5.65 |
| FPase | 1.65 ± 0.09 | 2.48 ± 0.20 | 2.15 ± 0.14 | 2.01 ± 0.12 | |
| Xylanase | 1609.00 ± 51.64 | 1958.46 ± 89.30 | 1805.33 ± 104.89 | 1689.26 ± 110.47 | |
| Soyabean | Endoglucanase | 78.12 ± 4.97 | 86.35 ± 6.97 | 92.52 ± 5.41 | 80.18 ± 5.51 |
| FPase | 1.43 ± 0.05 | 1.68 ± 0.09 | 1.70 ± 0.09 | 1.61 ± 0.08 | |
| Xylanase | 1205.20 ± 66.28 | 1512.68 ± 92.27 | 1769.52 ± 96.79 | 1694.20 ± 107.58 | |
| Tryptone | Endoglucanase | 88.40 ± 6.24 | 113.08 ± 7.28 | 115.13 ± 6.65 | 106.91 ± 6.35 |
| FPase | 1.64 ± 0.08 | 1.94 ± 0.11 | 1.88 ± 10 | 1.74 ± 0.13 | |
| Xylanase | 1294.11 ± 75.05 | 1390.43 ± 72.58 | 1412.65 ± 89.42 | 1680.62 ± 63.35 | |
| Yeast | Endoglucanase | 100.74 ± 5.73 | 119.24 ± 8.09 | 115.13 ± 5.69 | 111.02 ± 6.79 |
| FPase | 1.62 ± 0.11 | 1.93 ± 0.09 | 1.98 ± 0.09 | 1.70 ± 0.14 | |
| Xylanase | 1712.72 ± 133.07 | 1979.45 ± 121.73 | 2139.98 ± 97.58 | 2056.01 ± 141.45 | |
| Control | Endoglucanase | 76.01 ± 4.16 | |||
| FPase | 1.33 ± 0.08 | ||||
| Xylanase | 1289.66 ± 74.54 | ||||
Fermentation conditions (SSF): Incubation time – 4th day, temperature – 30 °C, pH – 8.0, moisture content – 80.0%, Carbon source – black gram residue, Results are presented as mean ± standard deviation.
Enzyme activities: FPase (FPU/gds), endoglucanase (IU/gds), xylanase (IU/gds).
3.3.6. Surfactants
Tween-20, Tween-40, Tween-60, Tween-80, and Triton-X-100 have stimulatory effects on cellulase and xylanase production while SDS and EDTA have inhibitory effect on cellulase and xylanase production (Table 2). Maximum endoglucanase (152.14 IU/gds) and FPase (3.42 FPU/gds) activities were obtained with 0.05% (w/v) Triton-X-100 while maximum xylanase (2491.41 IU/gds) production was observed with 0.1 (% w/v) Tween-80. Surfactants enhance microbial growth in SSF by promoting the penetration of water into solid substrate matrix which results into an increase in surface area of substrate [41]. Surfactants also increase the cell membrane permeability that would provoke a great release of proteins in culture broth. Another reason for enhanced production of enzymes may be release of cell-bound enzymes by surfactants [42]. It was also observed that Tween-20, Tween-40, Tween-60, Tween-80, and Triton-X-100 showed stimulatory effect between surfactant doses of 0.05–0.10% and thereafter, it showed inhibitory effect on enzyme production. Possibly, stimulatory effect may be inhibited due to poor spore germination using higher concentration of surfactant [43]. Present findings are in agreement with the observations of Deswal et al. [18] who reported maximum endoglucanase production (98.26 IU/gds) by Fomitopsis sp. on Triton-X-100 supplemented with wheat bran under SSF condition. Vu et al. [41] reported maximum cellulase production obtained from Aspergillus sp. SU14 (40 IU/gds) with addition of Tween-80. The endoglucanase, FPase, β-glucosidase and xylanase activities at optimum conditions were compared with endoglucanase, FPase, β-glucosidase and xylanase activities obtained with different carbon sources as reported by different researchers [18], [24], [25], [44], [45], [46], [47] (Table 3).
Table 2.
Effect of surfactants on enzyme production by Aspergillus nidulans AKB-25.
| Surfactants | Endoglucanase (IU/gds) |
FPase (FPU/gds) |
Xylanase (IU/gds) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.05% | 0.10% | 0.20% | 0.05% | 0.10% | 0.20% | 0.05% | 0.10% | 0.20% | |
| Tween-20 | 133.64 ± 7.10 | 139.75 ± 6.83 | 131.58 ± 8.05 | 2.75 ± 0.12 | 2.89 ± 0.14 | 2.64 ± 0.14 | 2028.10 ± 130.61 | 2221.72 ± 149.74 | 1830.53 ± 116.78 |
| Tween-40 | 133.64 ± 6.30 | 135.69 ± 7.25 | 123.36 ± 6.05 | 2.85 ± 0.13 | 2.69 ± 0.16 | 2.59 ± 0.14 | 2245.43 ± 133.37 | 2203.94 ± 138.18 | 2035.02 ± 98.29 |
| Tween-60 | 131.58 ± 9.00 | 143.92 ± 8.28 | 137.75 ± 8.07 | 2.58 ± 0.14 | 2.86 ± 0.11 | 2.54 ± 0.10 | 2124.91 ± 134.29 | 2380.77 ± 135.46 | 1970.80 ± 103.07 |
| Tween-80 | 145.97 ± 8.96 | 143.92 ± 9.28 | 125.41 ± 7.12 | 3.16 ± 0.16 | 2.86 ± 0.16 | 2.57 ± 0.14 | 2292.85 ± 130.46 | 2491.41 ± 146.49 | 2240.49 ± 108.21 |
| Triton-x-100 | 152.14 ± 8.29 | 145.97 ± 6.96 | 133.64 ± 6.61 | 3.42 ± 0.18 | 3.14 ± 0.20 | 3.20 ± 0.17 | 2441.03 ± 144.99 | 2128.86 ± 98.35 | 1648.76 ± 106.34 |
| SDS | 119.24 ± 6.98 | 108.96 ± 6.95 | 98.68 ± 3.99 | 2.59 ± 0.15 | 2.36 ± 0.13 | 2.05 ± 0.12 | 1669.50 ± 104.67 | 1473.16 ± 84.26 | 1334.86 ± 65.94 |
| EDTA | 117.19 ± 7.35 | 111.02 ± 5.90 | 94.57 ± 5.40 | 2.51 ± 0.10 | 2.25 ± 0.12 | 2.20 ± 0.13 | 1632.46 ± 78.84 | 1436.12 ± 98.37 | 1273.12 ± 70.14 |
| Control | 127.47 ± 6.90 | 2.66 ± 0.14 | 1743.49 ± 107.57 | ||||||
Fermentation conditions (SSF): Incubation time – 4th day, temperature – 30 °C, pH – 8.0, moisture content – 80.0%, Carbon source – black gram residue, nitrogen source – (NH4)2SO4 (0.12% as available nitrogen). Results are presented as mean ± standard deviation.
Table 3.
Comparison of FPase, endoglucanase, β-glucosidase and xylanase production by A. nidulans AKB-25 with other fungi under SSF.
| Organisms | Carbon sources | Enzyme activities (IU/gds) |
References | |||
|---|---|---|---|---|---|---|
| FPase | Endoglucanase | Glucosidase | Xylanase | |||
| Aspergillus ellipticus | Bagasse | 2.10 | 4.20 | 11.75 | – | [44] |
| Aspergillus fumigatus | Bagasse | 3.75 | 11.70 | 7.65 | – | [44] |
| Myceliophthora sp. | Rice straw | 2.44 | 32.9 | 7.48 | 900.2 | [45] |
| Aspergillus niger | Soybean meal | 0.55 | 35.1 | – | 47.7 | [24] |
| Fomitopsis sp. | Wheat bran | 4.68 | 81.82 | 69.08 | – | [18] |
| Aspergillus oryzae P27C3A | Wheat bran | 0.25 | 113.40 | 2.00 | 507.90 | [25] |
| Thermoascus aurantiacus | Jatropha seed cake | 4.87 | 124.44 | 28.86 | – | [46] |
| T. reesei Rut C-30 | Wheat bran | 0.80 | 142.1 | 1.20 | 768.4 | [25] |
| Aspergillus niger 3T5B8 | Wheat bran | 0.40 | 219.20 | 22.30 | 3382.50 | [25] |
| Trichoderma asperellum RCK2011 | Wheat bran | 2.2 | 13.1 | 9.2 | – | [47] |
| Aspergillus nidulans AKB25 | Black gram residue | 3.42 | 152.14 | 35.11 | 2441.03 | Present work |
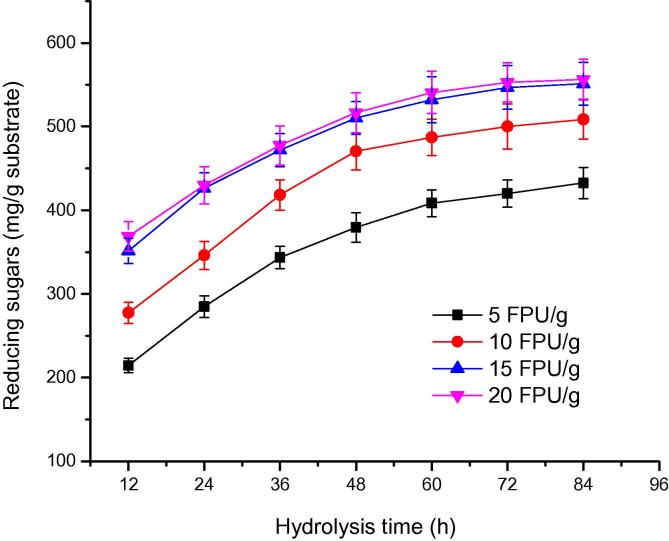
3.4. Saccharification of pearl millet stover
Pearl millet stover was selected as the raw material for the production of reducing sugars because of abundant availability. It is very difficult to obtain high yield of reducing sugars from all the lignocellulosic substrates without pretreatment because lignin present in the cell wall hinders the enzyme action [48]. The efficiency of crude enzyme A. nidulans AKB-25 having a mixture of cellulases (endoglucanase, FPase, β-glucosidase) and xylanase on saccharification of millet stover was investigated. The crude enzyme contained endoglucanase (14.19 IU/ml), FPase (0.36 FPU/ml), β-glucosidase (4.21 IU/ml), and xylanase (231.44 IU/ml) at pH 5.0 and temperature 50 °C. The presence of xylanase and β-glucosidase along with cellulase in the crude enzyme is essential for the hydrolysis of lignocellulosic biomass into fermentable sugars. Fig. 6 shows that reducing sugars production increased with increasing enzyme loading from 5 to15 FPU/g of pearl millet stover with respect to each hydrolysis time. The curves plotted between reducing sugars released during saccharification versus hydrolysis time at enzymes doses of 15 and 20 FPU/g coincide each other indicating that there was no increase in reducing sugars production at enzyme dose of 20 FPU/g of pearl millet stover. The production of reducing sugars increased with increasing hydrolysis time at different enzyme doses and beyond a hydrolysis time of 72 h it became almost constant. The maximum reducing sugars (546.91 mg/g of substrate) were obtained at enzyme dose of 15 FPU/g of substrate. At high enzyme dose, relative number of binding sites reduced and enzyme may start competing for same binding sites which may result reduction in overall rate [49]. Saha and Cotta [50] investigated the hydrolysis of rice hull by commercial enzyme (Celluclast 1.5 L and Novozyme 188) and reported reducing sugar level of 154 mg/g of the substrate. Deswal et al. [18] also studied the saccharification of wheat straw and observed reducing sugars production of 214.1 mg/g of the substrate by crude enzyme by Fomitopsis sp. RCK2010.
Figure 6.
Hydrolysis of pearl millet stover by crude enzyme from Aspergillus nidulans AKB-25 at temperature 50 °C, pH 5.0 for 96 h. Enzyme doses varied from 5 to 20 FPU/g of substrate.
3.5. Bio-deinking of MOW paper
The efficacy of crude enzyme from A. nidulans AKB-25 was also evaluated for deinking of MOW. The small pieces of MOW when pulped as per conditions described in Table 4 produced pulp of brightness of 59.8% (ISO) and dirt counts of 6017 mm2/m2. The pulp freeness, brightness, deinking efficiency and all the mechanical strength properties except tear index improved with increasing enzyme dose up to 3.0 IU/g compared to their respective control and thereafter there was not significant improvement in optical properties while mechanical strength properties started to decline (Table 4). Likewise, following the same pattern, maximum reduction in dirt counts was achieved at an enzyme dose of 3.0 IU/g. Crude enzymes obtained from A. nidulans AKB-25 showed an improvement in pulp freeness, brightness, deinking efficiency, burst index, tensile index and double fold by 34.47%, 4.6%, 25.01%, 13.68%, 12.81% and 14.28% and dirt counts and tear index mitigated by 74.70% and 9.30% respectively compared to respective control (Table 4). The pulp showed an increase in pulp freeness up to an enzyme dose of 3.0 IU/g of pulp and beyond that there was small increase in freeness. Reducing sugars in filtrate increased at all the enzyme doses applied during enzymatic treatment. Gubitz et al. [51] reported that an enzyme concentration of about 25 IU/g pulp results in a deinking efficiency of about 75%, while, Morkbak and Zimmermann [52] reported that the enzyme concentration of about 3.0 IU/g pulp is adequate to deink the mixed office waste papers, old newspapers and vegetable oil based ink printed papers. The selection of optimal enzyme dose is essential since excessive enzyme dose may be detrimental to fibre quality and strength properties due to deterioration of fibre and removal of microfibrils [11], [53], [54]. Due to the high specific surface area of the fines generated during pulping of MOW paper, the attack of cellulase is specific towards this fraction of the pulp. Therefore, the mechanism of degradation of carbohydrates is called as “peeling effect” [55]. The primary fines in recycled pulp have higher affinity towards water but do not contribute to interfibre bonding significantly. Cellulases acts preferentially on the fines and microfibrils protruding out the surface and removal of this fraction improves the freeness of the pulp. Moreover, preferential removal of fines improves the inter-fibrillar bonding which enhances the strength properties such as burst index and tensile index. An intensive enzymatic reaction leads to both intrinsic fibre strength and fibre length reduction, and excessive fines production. As a result, the paper strength will be drastically affected [11], [51], [53], [56], [57].
Table 4.
Effect of enzyme dose (A. nidulans AKB-25) on optical and strength properties of MOW.
| Particulars | Results after pulping (blank) | ||||
| CSF (ml) | 270 ± 5 | ||||
| Brightness (%) | 59.8 ± 0.2 | ||||
| Dirt count (mm2/m2) | 6017 ± 192 | ||||
| Results after ink flotation | |||||
| Enzyme dose (IU/g, OD pulp basis) | Control | 2.0 | 3.0 | 4.0 | 5.0 |
| CSF (ml) | 380 ± 3 | 430 ± 3 | 511 ± 5 | 530 ± 4 | 550 ± 5 |
| Brightness (%) | 68.3 ± 0.2 | 71.7 ± 0.3 | 72.9 ± 0.3 | 73.1 ± 0.2 | 73.2 ± 0.3 |
| Deinking efficiency (%) | 46.21 ± 1.1 | 64.70 ± 1.6 | 71.22 ± 1.5 | 72.31 ± 1.3 | 72.85 ± 1.6 |
| Dirt count (mm2/m2) | 2214 ± 62 | 652 ± 20 | 560 ± 14 | 514 ± 19 | 487 ± 17 |
| Burst index (kPam2/g) | 2.85 ± 0.22 | 2.96 ± 0.27 | 3.24 ± 0.17 | 2.90 ± 0.32 | 2.52 ± 0.20 |
| Tear index (mNm2/g) | 10.64 ± 0.85 | 10.28 ± 0.77 | 9.65 ± 0.68 | 9.04 ± 0.55 | 8.13 ± 0.71 |
| Tensile index (Nm/g) | 43.30 ± 1.73 | 47.01 ± 2.35 | 48.85 ± 2.88 | 44.53 ± 2.02 | 40.79 ± 2.25 |
| Double fold, numbers | 35 ± 4 | 36 ± 5 | 40 ± 4 | 36 ± 5 | 27 ± 4 |
| Reducing sugars (mg/ml) | – | 0.34 ± 0.02 | 0.51 ± 0.03 | 0.73 ± 0.05 | 1.10 ± 0.06 |
| Pulp yield (%) | 76.48 ± 0.78 | 75.74 ± 0.67 | 74.43 ± 0.54 | 73.28 ± 0.63 | 72.63 ± 0.65 |
Pulping conditions: Pulping time, min: 20, surfactant, % (w/w): 0.10, pH: 9.5 ± 0.2, consistency, %: 10, temperature, °C: 40.
Enzymatic treatment conditions: Reaction time, min: 60, enzyme dose, IU/g OD pulp: varied (2.0–5.0), pH: 5.0 ± 0.2, consistency, %: 10, temperature, °C: 50 ± 2.
Flotation conditions: Flotation time, min: 10, surfactant, % (w/w): 0.10, pH: 7.5 ± 0.2, consistency, %: 1.0, temperature, °C: 35 ± 2.
4. Conclusions
Black gram residue was found as most suitable carbon source for enzyme production by A. nidulans AKB-25. During screening of carbon sources and optimisation of cultural parameters endoglucanase, FPase, β-glucosidase and xylanase activities were improved by 5.7, 3, 1.1 and 1.8 times compared to the initial level of enzyme production. The crude enzyme from A. nidulans AKB-25 was successfully utilised in saccharification of pearl millet stover and bio-deinking of MOW paper. Crude enzyme hydrolysed alkali-pretreated pearl millet stover efficiently due to the presence of β-glucosidase and xylanase activities in the enzyme preparation. Pulp brightness, deinking efficiency, burst index, tensile index and double fold of mixed office waste paper were improved while dirt counts and tear index were mitigated after bio-deinking of mixed office waste using crude enzyme from A. nidulans AKB-25.
Acknowledgement
The authors are thankful to Jawaharlal Nehru Memorial Fund, Teen Murti House, New Delhi for awarding scholarship to carry out the present work.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Pathak H., Bhatia A., Jain N., Aggarwal P. In: ING Bulletins on Regional Assessment of Reactive Nitrogen, New Delhi. Singh B., editor. 2010. p. 34. [Google Scholar]
- 2.IIPR . Indian Institute of Pulses Research; Kanpur: 2011. Vision 2030; p. ix+42. [Google Scholar]
- 3.IARI . Indian Agricultural Research Institute; New Delhi: 2012. Crop Residues Management with Conservation Agriculture: Potential, Constraints and Policy Needs; p. vii+32. [Google Scholar]
- 4.Ilyas U., Ahmed S., Majeed A., Nadeem M. Afr. J. Biotechnol. 2012;11(38):9276–9279. [Google Scholar]
- 5.Kumar A., Gautam A., Dutt D. Adv. Biosci. Biotechnol. 2016;7(03):149–168. [Google Scholar]
- 6.Chandra M., Kalra A., Sharma P.K., Kumar H., Sangwan R.S. Biomass Bioenergy. 2010;34:805–811. [Google Scholar]
- 7.Vintila T., Croitoriu V., Dragomirescu M., Nica D. SPASB. 2010;43:337–340. [Google Scholar]
- 8.Pathak P., Bhardwaj N., Singh A. Appl. Biochem. Biotechnol. 2014;172:3776–3797. doi: 10.1007/s12010-014-0758-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y., Xu J., Yuan Z., Xu H., Yu Q. Bioresour. Technol. 2010;101:3153–3158. doi: 10.1016/j.biortech.2009.12.080. [DOI] [PubMed] [Google Scholar]
- 10.Lal M., Dutt D., Kumar A., Gautam A. Cell. Chem. Technol. 2015;49:471–483. [Google Scholar]
- 11.Pathak P., Bhardwaj N.K., Singh A.K. Appita J. 2014;67(4):291–301. [Google Scholar]
- 12.El-Hadi A.A., El-Nour S.A., Hammad A., Kamel Z., Anwar M. J. Radiat. Res. Appl. Sci. 2014;7(1):23–28. [Google Scholar]
- 13.Krogh K.R., Morkeberg A., Jorgensen H., Frisvad J.C., Olsson L. Appl. Biochem. Biotechnol. 2004;113–116:389–401. doi: 10.1385/abab:114:1-3:389. [DOI] [PubMed] [Google Scholar]
- 14.Farinas C.S., Loyo M.M., Junior A.B., Tardioli P.W., Neto V.B., Couri S. New Biotechnol. 2010;27:810–816. doi: 10.1016/j.nbt.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Ghose T.K. IUPAC. 1987;59:257–268. [Google Scholar]
- 16.Wood T.M., Bhat K.M. Methods Enzymol. 1988;160:87–112. [Google Scholar]
- 17.Bailey M.J., Biely P., Poutanen K. J. Biotechnol. 1992;23:257–270. [Google Scholar]
- 18.Deswal D., Khasa Y.P., Kuhad R.C. Bioresour. Technol. 2011;102:6065–6072. doi: 10.1016/j.biortech.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 19.Tappi Press; Technology Park, P.O. box 105113, Atlanta, GA-330348-5113, USA: 2007. TAPPI Test Methods. [Google Scholar]
- 20.Miller G.L. Anal. Chem. 1959;3:426–428. [Google Scholar]
- 21.Delabona P.dS., Pirota R.D.P.B., Codima C.A., Tremacoldi C.R., Rodrigues A., Farinas C.S. Biomass Bioenergy. 2012;37:243–250. [Google Scholar]
- 22.Gao J., Weng H., Zhu D., Yuan M., Guan F., Xi Y. Bioresour. Technol. 2008;99:7623–7629. doi: 10.1016/j.biortech.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Jabasingh S.A., Nachiyar C.V. Ind. Crops Prod. 2011;34:1564–1571. [Google Scholar]
- 24.Vitcosque G.L., Fonseca R.F., Rodriguez-Zuniga U.F.R., Neto V.B., Couri S., Farinas C.S. Enzyme Res. 2012;2012:1–9. doi: 10.1155/2012/248983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pirota R.D.P.B., Delabona P.S., Farinas C.S. Bioenergy Res. 2014;7:744–752. [Google Scholar]
- 26.Dhillon G.S., Oberoi H.S., Kaur S., Bansal S., Brar S.K. Ind. Crops Prod. 2011;34:1160–1167. [Google Scholar]
- 27.Kavya V., Padmavathi T. Pol. J. Microbiol. 2009;58:125–130. [PubMed] [Google Scholar]
- 28.Gautam S.P., Bundela P.S., Pandey A.K., Khan J., Awasthi M.K., Sarsaiya S. Biotechnol. Res. Int. 2011;2011:1–8. doi: 10.4061/2011/810425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaudhary K., Tauro P. World J. Microbiol. Biotechnol. 1986;2:399–403. [Google Scholar]
- 30.Sabu A., Sarita S., Pandey A., Bogar B., Szakacs G., Soccol C. Appl. Biochem. Biotechnol. 2002;102–103:251–260. doi: 10.1385/abab:102-103:1-6:251. [DOI] [PubMed] [Google Scholar]
- 31.Sun H., Ge X., Hao Z., Peng M. Afr. J. Biotechnol. 2010;9:163–166. [Google Scholar]
- 32.Fengel D., Wegener G. Walter de Gruyter; Berlin: 1983. Wood: Chemistry, Ultrastructure, Reactions. [Google Scholar]
- 33.Hendriks A.T.W.M., Zeeman G. Bioresour. Technol. 2009;100:10–18. doi: 10.1016/j.biortech.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 34.Mamma D., Kourtoglou E., Christakopoulos P. Bioresour. Technol. 2008;99:2373–2383. doi: 10.1016/j.biortech.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 35.Poorna C.A., Prema P. Bioresour. Technol. 2007;98:485–490. doi: 10.1016/j.biortech.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 36.Pandey A., Soccol C.R., Mitchell D. Process Biochem. 2000;35:1153–1169. [Google Scholar]
- 37.Lee C.K., Darah I., Ibrahim C.O. Biotechnol. Res. Int. 2011;2011:1–6. doi: 10.4061/2011/658493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stewart J.C., Parry J.B. J. Gen. Microbiol. 1981;125:33–39. doi: 10.1099/00221287-125-1-33. [DOI] [PubMed] [Google Scholar]
- 39.Kalogeris E., Christakopoulos P., Katapodis P., Alexiou A., Vlachou S., Kekos D., Macris B.J. Process Biochem. 2003;38:1099–1104. [Google Scholar]
- 40.Vyas A., Vyas D., Vyas K.M. J. Sci. Ind. Res. 2005;64:281–286. [Google Scholar]
- 41.Vu V.H., Pham T.A., Kim K. Mycobiology. 2011;39:20–25. doi: 10.4489/MYCO.2011.39.1.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pardo A. Curr. Microbiol. 1996;33:275–278. doi: 10.1007/s002849900113. [DOI] [PubMed] [Google Scholar]
- 43.Chellapandi P., Jani A.A. Turk. J. Biochem. 2009;34:209–214. [Google Scholar]
- 44.Gupte A., Madamwar D. Biotechnol. Progr. 1997;13:166–169. [Google Scholar]
- 45.Badhan A.K., Chadha B.S., Kaur J., Saini H.S., Bhat M.K. Bioresour. Technol. 2007;98:504–510. doi: 10.1016/j.biortech.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Dave B.R., Sudhir A.P., Parmar P., Pathak S., Raykundaliya D.P., Subramanian R.B. Biocatal. Agric. Biotechnol. 2013;2:108–115. [Google Scholar]
- 47.Raghuwanshi S., Deswal D., Karp M., Kuhad R.C. Fuel. 2014;124:183–189. [Google Scholar]
- 48.Jeya M., Zhang Y.-W., Kim I.-W., Lee J.-K. Bioresour. Technol. 2009;100:5155–5161. doi: 10.1016/j.biortech.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 49.Dyk J.S.V., Pletschke B.I. Biotechnol. Adv. 2012;30:1458–1480. doi: 10.1016/j.biotechadv.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Saha B.C., Cotta M.A. Biomass Bioenergy. 2008;32:971–977. [Google Scholar]
- 51.Gubitz G.M., Mansfield S.D., Böhm D., Saddler J.N. J. Biotechnol. 1998;65:209–215. [Google Scholar]
- 52.Mørkbak A.L., Zimmermann W. Prog. Pap. Recycl. 1998;7:14–21. [Google Scholar]
- 53.Lee C.K., Darah I., Ibrahim C.O. Bioresour. Technol. 2007;98(8):1684–1689. doi: 10.1016/j.biortech.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 54.Pathak P., Bhardwaj N.K., Singh A.K. Nord. Pulp Pap. Res. J. 2015;30(4):689–700. [Google Scholar]
- 55.Julien S.A.F., Perrin B., editors. The 3rd Advanced Training Course on Deinking Technology Course Note. Centre Technique, du Papier; 1997. [Google Scholar]
- 56.Dienes D., Egyházi A., Réczey K. Ind. Crops Prod. 2004;20(1):11–21. [Google Scholar]
- 57.Vyas S., Lachke A. Enzyme Microb. Technol. 2003;32(2):236–245. [Google Scholar]