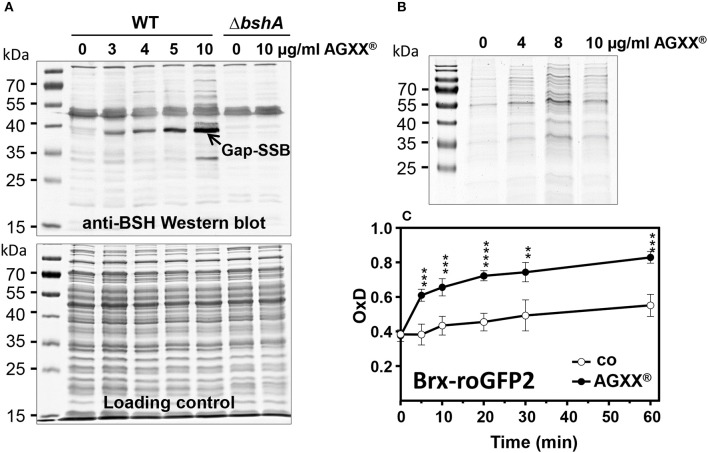
Figure 4.
AGXX® causes protein S-bacillithiolation, protein aggregation and an oxidative shift in the BSH redox potential in S. aureus. (A) Non-reducing BSH-specific Western blot analysis showed increasing of S-bacillithiolated GapDH protein in S. aureus USA300 strain under AGXX® stress. The Coomassie-stained SDS-PAGE is shown as loading control below the BSH Western blot. (B) To analyze protein aggregates, S. aureus cells were grown in RPMI medium to OD500 of 0.5 and treated with 4–10 μg/ml AGXX® for 30 min. The insoluble protein fractions of protein aggregates were obtained by centrifugation of the cell lysates and repeated centrifugation of the NP-40-dissolved pellet as described in the Methods section. The insoluble protein fractions were analyzed by SDS-PAGE and visualized with Coomassie staining (C) S. aureus COL expressing Brx-roGFP2 was treated with the sub-lethal amount of 4 μg/ml AGXX® at OD500 of 0.5 in RPMI medium and the biosensor oxidation degree (OxD) was measured at different times after stress. The Brx-roGFP2 biosensor is rapidly oxidized by AGXX®. The OxD values of the Brx-roGFP2 biosensor measurements with and without AGXX® were calibrated to fully reduced (DTT-treated) and oxidized (diamide-treated) control samples. The OxD values of fully reduced and oxidized controls were defined as “0” and “1,” respectively. The data were calculated as mean values from 3 biological replicate experiments. Error bars represent the SD and p-values are calculated using a Student's unpaired two-tailed t-test by the graph prism software. Symbols are defined as follows: **p ≤ 0.01; ***p ≤ 0.001 and ****p ≤ 0.0001 (p < 0.0003 for control/AGXX stress at 5 min; p = 0.0002 at 10 min; p < 0.0001 at 20 min; p = 0.0013 at 30 min; p = 0.0001 at 60 min).