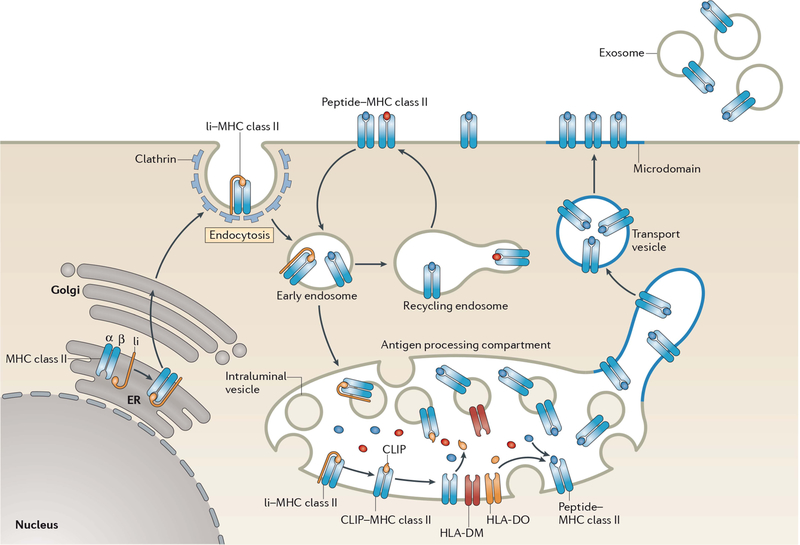
FIGURE 2.
Biosynthesis of MHC-II–peptide complexes. MHC-II αβ dimers associate with Ii in the ER, and the assembled MHC-II–Ii complexes traffic through the Golgi apparatus and are delivered to the plasma membrane. The complexes are internalized by clathrin-mediated endocytosis and are transported to late endosomal multivesicular antigen-processing compartments. Some of these complexes sort onto the ILVs of these compartments, where sequential Ii proteolysis leads to persistence of a derived fragment (termed CLIP) in the MHC-II peptide-binding groove. CLIP is removed from CLIP–MHC-II complexes by DM molecules that are present on the ILV and limiting membrane of antigen-processing compartments, thereby allowing peptide binding onto nascent MHC-II. The activity of DM is regulated by DO; however, the mechanism of regulation remains unknown. It is likely that ILV-associated MHC-II is transferred to the limiting membrane and endo/lysosomal tubules that either directly fuse, or give rise to transport vesicles that fuse, with the plasma membrane. MHC-II–peptide association with lipid microdomains first occurs in antigen-processing compartments and allows clustering of MHC-II–peptide complexes on the cell surface. If an entire antigen-processing compartment fuses with the plasma membrane, the ILV can be released from the cell in the form of exosomes. Surface-expressed MHC-II–peptide complexes can internalize using a clathrin-independent endocytosis pathway and are targeted for lysosomal degradation or may be recycled back to the plasma membrane. Reprinted from reference 103, with permission.