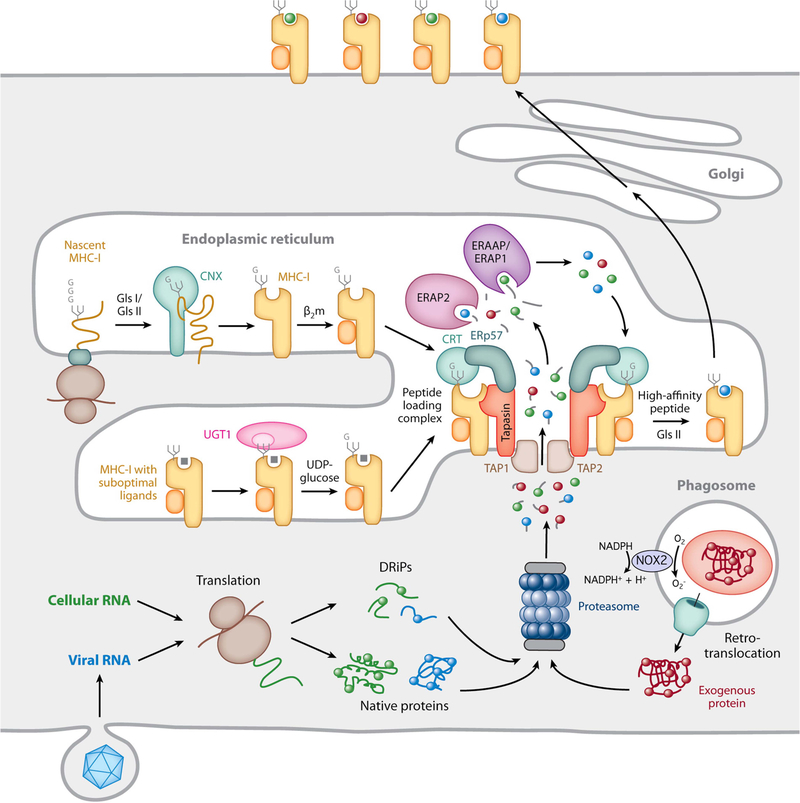
FIGURE 3.
MHC-I biosynthesis and peptide binding. The proteasome generates short antigenic peptides capable of binding to MHC-I molecules. These peptides are derived from native cytosolic proteins, defective ribosomal products (DRiPs), or, in the case of cross-presentation, exogenous proteins that enter the cell by phagocytosis and are translocated into the cytosol, either intact or as large proteolytic fragments. In cross-presenting mouse CD8+ DCs, the presence of NOX2 on the phagosomal membrane neutralizes acidification and reduces proteolytic activity, preserving protein integrity. Nascent MHC-I heavy chains initially interact with the molecular chaperone calnexin (CNX) and, after binding β2m, are recruited to the PLC by simultaneous noncovalent CRT interactions with a monoglucosylated N-linked glycan on the heavy chain and ERp57 disulfide linked to tapasin in the PLC. Peptide-free MHC-I molecules and those possessing suboptimal ligands are subject to a series of “editing” steps mediated by interaction with tapasin within the PLC as well as maintenance of the monoglucosylated N-linked glycan by the opposing actions of the enzymes glucosidase 2 (GlsII), which removes the terminal glucose residue, and UGT1, which adds back glucose to preserve the CRT interaction. MHC-I molecules containing high-affinity peptides ultimately leave the ER and are transported to the plasma membrane. Reprinted from reference 32, with permission.