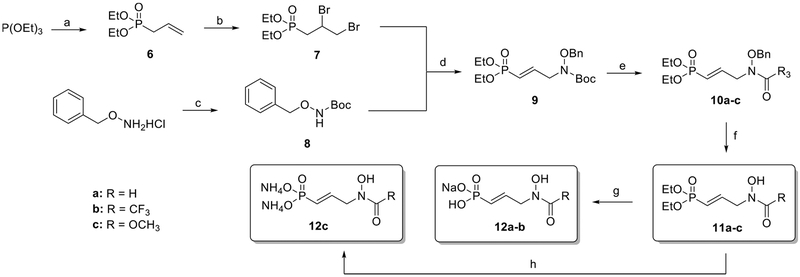
Scheme 1. Synthesis of N-Acyl Analogues 11a–c, 12a–ca.
aReagents and conditions: (a) allyl bromide, 60 °C, 2 days (85%); (b) Br2, CH2Cl2, 0 °C to rt, 2 h (88%); (c) Boc2O, TEA, H2O, THF, rt, 2.5 h (90%); (d) NaH, NaI, THF, 0 °C to rt, 20 h (77%); (e) (i) AcCl, MeOH, rt, 30 min, (ii) Na2CO3, RCOCl or (RCO)2O, 0 °C to rt, 30 min to 24 h (10a: Na2CO3, HCOOH, 1,1′-carbonyldiimidazole, 0 °C, 30 min) (50–73%); (f) BCl3, CH2Cl2, −70 °C, 30 min to 3 h (19–85%); (g) (i) TMSBr, CH2Cl2, 0 °C to rt, 24 h, (ii) NaOH, H2O, rt, 1 h (quant); (h) (i) TMSBr, CH2Cl2, 0 °C to rt, 24 h, (ii) NH4OH, H2O, rt, 1 h (quant).