Abstract
Discrimination of similar spatial locations, an important feature of episodic memory, has traditionally been measured via delayed nonmatching-to-location tasks. Recently, we and others have demonstrated that touchscreen-based Trial Unique Nonmatching-to-Location (TUNL) tasks are sensitive to lesions of the dorsal hippocampus in the mouse. Previously we have shown that loss of the GluN2B subunit of the N-methyl-D-aspartate (NMDA) receptor in the dorsal CA1 and throughout the cortex impairs hippocampal-dependent water maze and fear conditioning paradigms. We investigated whether loss of GluN2B would alter performance of visual-spatial discrimination learning in a delay- or separation-dependent manner. GluN2B null mutants displayed initial impairments in accuracy on the easiest training variant of TUNL that were overcome with training. Loss of GluN2B also impaired performance on a problem series where delay and separation were systematically varied. We also observed a training-dependent effect on performance. Mutant mice that received extensive training performed similar to control mice when challenged on a variable delay and variable separation problem, while those that received minimal training were impaired across all delays and separations. Together, these data demonstrate that GluN2B in the dorsal CA1 and cortex are essential for efficient visual-spatial discrimination learning on the TUNL task. Further, training effects on performance in mutant mice suggest that alterations in synaptic plasticity after GluN2B loss may underlie intra- versus inter-session learning.
1. Introduction
The ability of animals to encode distinct stimuli within the environment and then discriminate between them is crucial to survival and depends on a properly functioning hippocampus (S. Leutgeb & Leutgeb, 2007; MacDonald, Carrow, Place, & Eichenbaum, 2013; Sloan, Dobrossy, & Dunnett, 2006; White, 2004). Loss of hippocampal function has been linked with both neurodegenerative and neuropsychiatric disorders, such as Alzheimer’s disease and schizophrenia, as well as early life toxic exposures, which has made it a well-studied structure in biomedical research (Brady, Allan, & Caldwell, 2012; Mu & Gage, 2011; Small, Schobel, Buxton, Witter, & Barnes, 2011). Traditional assessments of hippocampal functioning have relied on maze-based tasks, e.g. Morris water maze, and aversive learning tasks, e.g. fear conditioning (Saxe et al., 2006; Vorhees & Williams, 2014). While these paradigms are well validated, the stress they cause rodents (i.e. cool water or aversive shock) may possibly confound results. Additionally, such assays are not tenable in a clinical setting. Other tests utilizing operant learning approaches may be more relevant to clinical approaches. For example, the delayed nonmatching-to-position task (DNMTP) tests an animal’s ability to discriminate between two spatially discrete locations and has been shown to be sensitive to hippocampal dysfunction in rodents (Aggleton, Kentridge, & Sembi, 1992). However, DNMTP and similar tasks may have limited translational potential as they are typically not challenging enough for human subjects due to the manipulation of only one dimension (delay period presentations). Additionally, they have also been shown to be vulnerable to mediating behaviors in rodents, such as body positioning, which can decrease their value as measures of spatial discrimination.
Recently, a touchscreen-based automated operant approach has been developed for use in mice in an attempt to bypass the problems posed by traditional methods (Bussey et al., 2012; Oomen et al., 2013). These paradigms, referred to as Trial Unique Nonmatching-to-Location (TUNL) tasks have been adapted and modified to assess both spatial- and delay-dependent hippocampal memory in mouse models of hippocampal dysfunction (Josey & Brigman, 2015; Kim et al., 2015; Oomen et al., 2015). While these paradigms are sensitive to loss of global dorsal hippocampal function or lesions restricted to the dentate gyrus in rodents, the mechanisms underlying trial-to-trial discrimination of spatial locations in these tasks is still not fully (Santoro, 2013).
The N-methyl-D-aspartate receptor (NMDAR) has previously been shown to be involved in learning and memory, as well as synaptic plasticity (Hunt & Castillo, 2012; Volianskis et al., 2015). GluN2B is the dominant NMDAR subunit in the hippocampus and cortex throughout development (Cull-Candy, Brickley, & Farrant, 2001; Marquardt, Saha, Mishina, Young, & Brigman, 2014) and GluN2B-containing NMDARs are posited to play a unique role in allowing the induction of plasticity, including long-term potentiation (LTP) necessary for optimal learning and memory across numerous paradigms (Brigman et al., 2015; Brigman et al., 2013; Brigman et al., 2010; France et al., 2017; Howland & Cazakoff, 2010; Shipton & Paulsen, 2014). While early global GluN2B knockdown is neonatally lethal, electrophysiological studies after forebrain knockout found neonates to have deficient hippocampal long-term depression (Kutsuwada et al., 1996). Previously, we utilized a targeted GluN2B knockout via a Cre/LoxP-based system to show that loss of GluN2B in the cortex and dorsal CA1 led to faster decay of NMDAR-mediated excitatory post-synaptic currents and impaired long-term depression in hippocampal principal neurons (Brigman et al., 2010). Importantly, these functional alterations were accompanied by impaired learning both in the Morris water maze and in a trace fear conditioning paradigm.
In the current experiment, we tested GluN2B cortico-hippocampal null mutant mice on variants of the TUNL paradigm that measured the ability to distinguish between spatial locations that varied in difficulty based on their similarity, as well as hold locations in working memory over varying delays. The use of the TUNL task that closely mimics clinical assessment tools such as the Cambridge Neuropsychological Test Automated Battery (CANTAB) allows us to examine the involvement of NMDAR function and cortical and CA1 contribution to hippocampal learning and memory that is compromised in various neuropsychiatric diseases and disorders.
2. Materials and Methods
2.1. Subjects.
GluN2B mutant mice were generated as previously described (Brigman et al., 2010). Briefly, the GluN2B gene was disrupted by inserting a loxP site downstream of the 599 bp exon 3 or exon 5 (depending on transcript) and a neomycin resistance gene cassette flanked by 2 loxP sites upstream of this exon. The 129/SvEvTac was used as the embryonic stem cell donor and C57BL/6J was used for blastocysts and as the genetic background for backcrossing. Analysis of 150 single nucleotide polymorphism markers at 15 to 20 Mb intervals estimated the genetic background of the mutant cross to be 95% C57BL/6J. GluN2BFLOX mice were crossed with (C57BL/6J-congenic) transgenic mice expressing Cre recombinase driven by the CaMKII promoter (T29–1 line). Cre+ hemizygous GluN2BFLOX (i.e., GluN2B excised) mice were crossed with Cre-GluN2BFLOX (non-excised controls) mice to produce mutant (GluN2BNULL) and control littermates for experimentation. Mice were housed in groupings of 2–4 per cage in a temperature and humidity-controlled vivarium under a reverse 12 h light/dark cycle (lights off 0800 h) and tested during the dark phase. Mice were aged 8 weeks at the onset of behavioral testing. All experimental procedures were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the University of New Mexico Health Sciences Center Institutional Animal Care and Use Committee.
2.2. Operant apparatus.
All operant behavior was conducted in a chamber measuring 21.6 × 17.8 × 12.7cm (model # ENV-307W, Med Associates, St. Albans, VT, USA) housed within a sound- and light-attenuating box (Med Associates) as previously described (Brigman et al., 2013; Marquardt, Sigdel, & Brigman, 2017). The standard grid floor of the chamber was covered with a solid acrylic plate to facilitate ambulation. A pellet dispenser delivering 14 mg dustless pellets (#F05684, BioServ, Frenchtown, NJ, USA) into a magazine, a house-light, tone generator and an ultra-sensitive lever was located at one end of the chamber. At the opposite end of the chamber there was a touch-sensitive screen (Conclusive Solutions, Sawbridgeworth, UK) covered by a black acrylic aperture plate allowing 2 rows of 5 touch areas measuring 2.5 × 2.5 cm separated by 0.6 cm and located at a height of 1.6 cm from the floor of the chamber. Stimulus presentation in the response windows and touches were controlled and recorded by the K-Limbic Software Package (Conclusive Solutions, Sawbridgeworth, U.K.).
2.3. Pretraining.
Mice were reduced and then maintained at 85% free-feeding body weight. Prior to testing, mice were acclimated to the 14 mg pellet food reward (BioServ, Flemington, NJ) by provision of ~10 pellets/mouse in the home cage for 3–5 days. After becoming acclimated to the reward, mice were then habituated to the operant chamber and eating out of the pellet magazine by being placed in the chamber for 30 min with 10 pellets available. Mice retrieving 10 pellets within 30 min were moved to a pre-training regimen. First, mice were able to obtain reward by pressing a lever within the chamber. Mice pressing and collecting 30 rewards in under 30 min were moved to touch training. In touch training, a lever press led to the presentation of a white square stimulus in 1 of 10 response windows (2.5 cm2; spatially pseudorandomized). The stimulus remained on the screen until a response was made and touches in blank response windows had no response. Criterion for touch training was touching, retrieving and eating 30 pellets within 30 min.
2.4. Trial-unique nonmatching-to-location.
Following pre-training mice were tested on the TUNL paradigm as previously described (J. C. Talpos, S. M. McTighe, R. Dias, L. M. Saksida, & T. J. Bussey, 2010). Briefly, mice lever pressed to initiate the onset of a trial. In the sample phase, 1 of the 10 response locations illuminated with a white square. Mice were required to nose poke the illuminated square in order to complete the sample phase (Fig. 1A). Thirty-three percent of sample phase responses were rewarded to ensure motivation. After an inter-phase-interval (IPI) delay period, which varied across problems, mice were required to lever press a second time to initiate the choice phase. In the choice phase, the stimulus from the sample phase and a stimulus in a novel location were illuminated concurrently. A touch at the novel stimulus resulted in delivery of a food reward and concomitant onset of the magazine light and a 1 sec tone. A touch at the previously presented stimulus resulted in a 15 sec house light-on timeout period before a new trial could be initiated. To aid learning and extinguish position bias, incorrect responses were followed by correction trials in which the same stimulus configurations were repeated until a correct response sequence was made. Correct trials were followed by a 5 sec inter-trial interval before the next trial could be initiated by lever press. Mice were tested daily for a maximum of 60 first presentation trials or 2 hours, whichever came first. Throughout testing, all 10 response locations were utilized and touches at nonilluminated windows during any phase had no response.
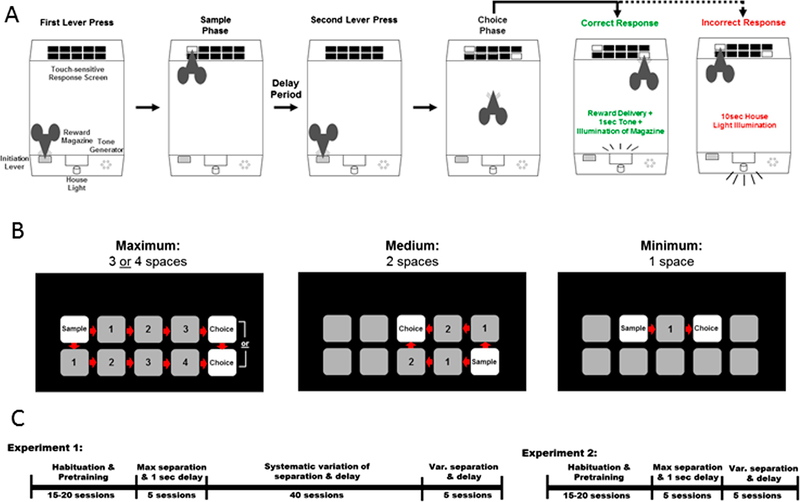
Fig. 1: Trial Unique Nonmatching-to-Location Paradigm schematic and stimuli separation diagrams:
(A) Mice lever press to initiate the onset of a trial. In the sample phase, mice must nose poke 1 of the 10 response locations illuminated with a white square. After an inter-phase-interval (IPI) delay period, mice were required to lever press a second time to initiate the choice phase. A touch at the novel stimulus resulted in reward and concomitant onset of the magazine light and a 1 sec tone. A touch at the previously presented stimulus resulted in a 15 sec house light-on timeout period before a new trial could be initiated. (B) Examples of one trial type for each of three stimulus separation difficulties: maximum (3–4 spaces), medium (2 spaces), or minimum (1 space) separation. (C) Schematic of design and timeframe of Experiments 1 and 2.
2.5. Experiment 1.
A cohort of GluN2BNULL and GluN2BFLOX controls (GluN2BNULL=12, GluN2BFLOX=10) were tested following a previously published design (Josey & Brigman, 2015; Fig 1C). Mice were first tested for 5 consecutive sessions on a problem of the task with a large separation (3–4 spaces between stimuli) and a minimum IPI delay (1 second; Fig. 1B). Mice were then tested on a decreased separation (2 spaces) and finally a minimum separation (1 space) for 5 consecutive sessions each. Following completion of testing on the three distinct separations, mice were then tested on an increasing IPI delays. Mice completed 5 sessions each of a consistent 6 sec and 12 sec IPI on maximum (3–4) medium (2) and minimum (1) square separation. Finally, mice were tested on a challenge problem that incorporated a variable IPI (1 sec/6 sec/12 sec) and variable separation (3–4 spaces/2 spaces/1 space).
2.6. Experiment 2.
Given the extensive training conducted in GluN2BNULL and GluN2BFLOX control mice prior to testing on the variable problem, a second cohort of mice (GluN2BNULL=8, GluN2BFLOX=8) was first trained on the maximum (3–4 space) separation with a minimum (1 sec) delay for 5 consecutive sessions (Fig. 1C). Mice were then immediately tested for 5 consecutive sessions on the challenge problem that incorporated a variable IPI (1 sec/6 sec/12 sec) and variable separation (3–4 spaces/2 spaces/1 space). To investigate how baseline motor activity may impact TUNL performance, home cage activity was then measured in a non-aversive environment (Sharma et al., 2013). Mice were individually housed in a standard cage with ad libitum food and water and left undisturbed for a 24-hour acclimation period. Horizontal activity was then automatically measured and recorded for 48 hrs by photocell beam break using the PAS-Homecage system (San Diego Instruments, San Diego, CA). After the total 72-hour period, mice were paired again with their cage mates and returned to their normal cages.
2.7. Statistical analysis.
The following dependent measures were taken during each phase of TUNL testing: Accuracy (correct responses/first presentation trials attempted), Correction Trials, Sample Phase Stimulus Response (time from trial initiation to touchscreen response), Choice Phase Stimulus Response (time from trial initiation of choice phase to touchscreen response), and Reward Response (time from correct choice phase touch to reward retrieval). Main effects and interactions were compared for all measures using repeated measures analysis of variance (ANOVA) with session as within factor and genotype and delay/separation (when applicable) as between factors. Group differences were then analyzed via Fisher’s PLSD post hoc tests.
3. Results
3.1. Experiment 1.
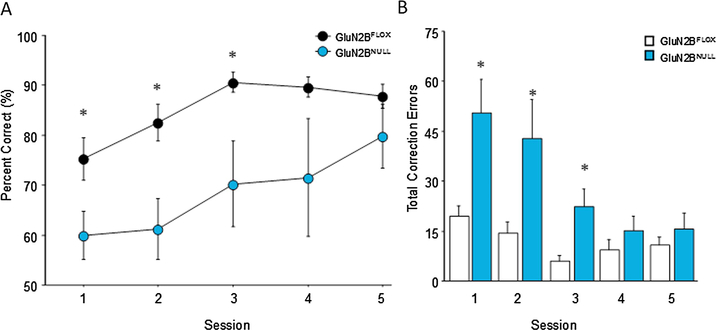
Analysis of performance on the easiest variant with maximum separation (3–4 spaces) and minimum delay (1 sec) found a significant main effect of session (F4,56=6.71; p=.0003) and a significant main effect of genotype (F1,56=5.74; p=.0311) with no significant interaction (F4,56=.812; p=.5209). Post hoc tests revealed that GluN2BNULL mice had significantly reduced accuracy on the first three sessions of the problem but were equivalent to GluN2BFLOX controls thereafter (Fig. 2A). Analysis of perseverative responding as measured by correction trials similarly found a significant main effect of session (F4,56=12.58; p=.0001) and a significant main effect of genotype (F1,56=7.09; p=.0186) as well as a significant session x genotype interaction (F4,56=43.830; p=.0067). Fisher’s PLSD revealed that GluN2BNULL mice initially had significantly increased perseveration during the first three sessions of the problem but were not different from GluN2BFLOX levels by session 4 and 5 of the initial problem (Fig. 2B). Analysis of secondary response measures revealed no significant differences in response latencies during either the sample (GluN2BFLOX=23.26±1.98 GluN2BNULL=23.07±1.99; t(14)=.068, p=.9466) or choice phase (GluN2BFLOX=6.22±.94 GluN2BNULL=8.24±1.53; t(14)=1.178, p=.2525) and no significant differences in their latency to retrieve reward after a correct response (GluN2BFLOX=2.07±.14 GluN2BNULL=2.95±.46; t(20)=1.668, p=.1108).
Fig. 2: Initial deficits in GluN2BNULL performance on an easy TUNL variant recover with sufficient training:
(A) GluN2BNULL mice were significantly impaired in performance on the first three sessions of the maximum separation, 1 sec delay task, but performed similar to controls on the fourth and fifth sessions. (B) GluN2BNULL mice also made significantly more correction errors than GluN2BFLOX during the first three sessions compared to controls but decreased to control levels by the final two sessions. Glun2BNULL=12, GluN2BFLOX=10; * = p<0.001. Data are shown as Mean±SEM.
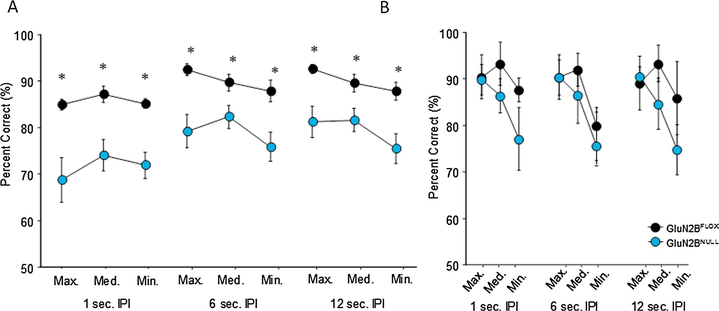
When the separation between stimuli and the IPI delay were systematically manipulated, significant differences between genotypes emerged as task demands increased. Analysis of average accuracy across sessions on maximum (3–4 spaces), medium (2 space), and minimum (1 space) separation with a 1 sec IPI found a significant main effect of genotype (F1,40=16.00; p=.0007) but no main effect of separation (F2,40=1.58; p=.2181) and no interaction (F2,40=0.35; p=.7049) indicating GluN2BNULL mice performed more poorly at every stage, but separation did not affect performance during short delays (Fig. 3A). When separations were tested systematically at a 6 sec IPI, a significant main effect of genotype (F1,40=10.11; p=.0049) and separation (F2,40=3.57; p=.0380) were seen with no interaction (F2,40=1.09; p=.3467). All mice performed more poorly as separation decreased, and GluN2BNULL mice showed decreased accuracy on all separations. Similarly, when IPI was increased to 12 sec, a main effect of genotype (F1,40=10.89; p=.0036) and separation (F1,56=6.11; p=.0048) and no interaction (F2,40=1.062; p=.3553) was seen with GluN2BNULL performance lower at every stage and a reduction in accuracy in all mice as separations decreased. There were no significant differences on latency to touch in the sample or choice phase, or latency to retrieve reward on any of the delay or separation problems.
Fig. 3: Systematic variation of delay and separation reveal consistent deficits in GluN2BNULL:
(A) When tested on systematic but fixed separations and delays, GluN2BNULL mice display significant reductions in performance relative to controls at all levels versus floxed controls. (B) GluN2BNULL mice do not show impaired performance on a difficult challenge task with variable separation and delay after following extensive testing on the TUNL paradigm. Glun2BNULL=12, GluN2BFLOX=10; * = p=0.001. Data are shown as Mean±SEM.
Analysis of a variable IPI delay and separation challenge problem revealed that there was a significant main effect of separation on the challenge problem (F2,108=5.49; p=.0054). However, there was no main effect of delay (F2,108=.183; p=.8715) and the effect of genotype did not reach significance (F1,108=3.83; p=.0529; Fig. 3B). No significant interactions were seen for genotype x separation (F2,108=1079; p=.3435), genotype x delay (F2,108=0.124; p=.8832), separation x delay (F4,108=0.099; p=.9826) or genotype x separation x delay (F4,108=0.073; p=.9902). No main effect of genotype was found on response latency during the choice (GluN2BFLOX=2.91±0.42 GluN2BNULL=3.75±0.34; t(20)=1.56, p=.1352) or sample phase (GluN2BFLOX=11.40±2.68 GluN2BNULL=11.58±0.99; t(20)=0.68, p=.9468) or their latency to retrieve reward (GluN2BFLOX=6.93±4.18 GluN2BNULL=2.94±0.49; t(20)=0.915; p=.3767) after a correct response during any of the variable durations.
3.2. Experiment 2.
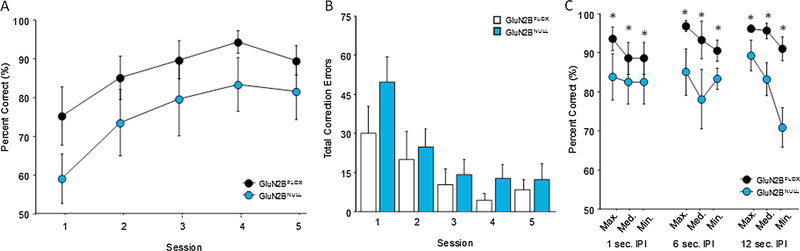
Performance on the easiest variant with maximum separation (3–4 spaces) and minimum delay (1 sec) was similar to cohort 1, with a significant main effect of session (F4,56=16.46; p=.0001). While GluN2BNULL mice had a reduced accuracy across each session of the problem, the main effect of genotype did not reach significance (F1,56=1.74; p=.2067; Fig. 4A) and there was no significant session x genotype interaction (F4,56=38.54; p=.7264). Analysis of perseverative responding as measured by correction trials similarly found a significant main effect of session (F4,56=14.07; p=.0001) but no significant main effect of genotype (F1,56=1.04; p=.3254; Fig. 4B) or session x genotype interaction (F4,56=0.961; p=.4361). Analysis of secondary response measures revealed no significant differences in response latencies during either the sample (GluN2BFLOX=12.76±1.17 GluN2BNULL=20.04±4.50; t(20)=.068, p=.9466) or choice phase (GluN2BFLOX=6.22±.94 GluN2BNULL=8.24±1.53; t(20)=1.178, p=.2525) and no significant differences in their latency to retrieve reward after a correct response (GluN2BFLOX=2.34±.35 GluN2BNULL=2.39±.17; t(20)=0.008, p=.9941).
Fig. 4: GluN2BNULL mice minimally trained on TUNL display impairments on the challenging variable delay and separation task:
(A) GluN2BNULL mice display non-significant reductions in performance relative to controls in the initial task with a maximum separation and a 1 sec delay. (B) GluN2BNULL mice show a non-significant increase in the number of errors made during early sessions of the initial task. (C) GluN2BNULL mice tested on variable delay and separation challenge task following training perform significantly worse than controls on trials with sufficiently close separations across all delays. Glun2BNULL=8, GluN2BFLOX=8; * =p=.001 Data are shown as Mean±SEM.
When mice were then immediately tested on the variable IPI and separation, ANOVA found a significant main effect of separation (F2,126=3.19; p=0.044) but no main effect of delay (F1,40=0.15; p=.8606; Fig. 4C). In addition, there was a main effect of genotype (F1,126=26.22; p=.0001) with Fisher’s post-hoc tests showing that GluN2BNULL had reduced accuracy at all separations and delays. No significant interactions were seen for genotype x separation (F2,126=.072; p=. 9303), genotype x delay (F2,126=0.700; p=.4986), separation x delay (F4,126=0.911 p=.4599) or genotype x separation x delay (F4,126=0.802; p=.5259). Analysis of secondary measures revealed no main effect of genotype on response latency during the sample (GluN2BFLOX=10.15±1.85 GluN2BNULL=11.69±2.49; t(14)=0.25; p=.6273) or choice phase (GluN2BFLOX=3.33±0.27 GluN2BNULL=4.41±0.72; t(14)=1.95; p=.1835) or their latency to retrieve reward (GluN2BFLOX=2.00±0.21 GluN2BNULL=2.30±0.27; t(14)=0.79; p=.3881) after a correct response during any of the variable durations.
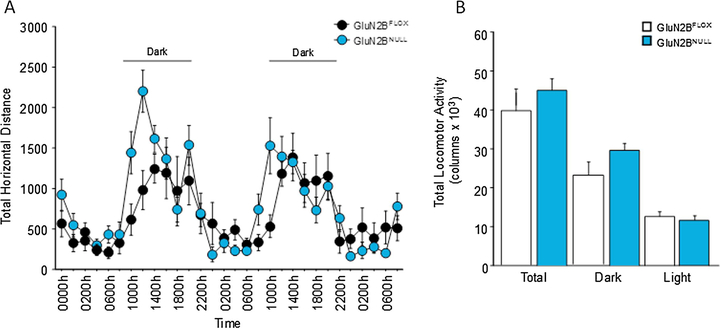
When tested for home cage activity, GluN2BNULL mice showed a non-significant increase in activity during the 1st dark phase (t(14)=2.19, p=.052) and similar rates of horizontal activity during the 2nd dark phase (t(14)=1.08, p=.294; Fig. 5A). No significant differences were seen in total activity either during all dark phases (t(14)=1.69, p=.113), light phases (t(14)=0.65, p=.522) or over the entire recording period (t(14)=0.99, p=.333; Fig. 5B).
Fig. 5: GluN2BNULL mice show no significant changes in activity in a familiar environment:
(A) GluN2B mice showed slight increases in activity during the initial dark phase but no significant differences in total locomotor activity across 48 hours or home cage behavior. (B) Analysis of activity by phase revealed no significant differences between GluN2BNULL and GluN2BFLOX controls during either the light or the dark phases. Glun2BNULL=8, GluN2BFLOX=8; Data are shown as Mean±SEM.
4. Discussion
In the current study, we found that cortical and hippocampal loss of GluN2B-containing NMDARs on principal neurons impaired the ability to discriminate between visual spatial stimuli on a mouse touchscreen TUNL task. By utilizing two different training approaches, we found that GluN2BNULL mice showed initial impairments in performance of an easy variant but could attain control-level performance with sufficient training. Systematic testing that varied separation and delay in a fixed manner showed that GluN2BNULL had significantly reduced accuracy for each separation tested but intact performance when tested on variable delays and separations within the same challenge session. In contrast, when tested on the variable challenge session immediately after training on the easiest variant, a separate cohort of GluN2BNULL were consistently impaired across delays and separations. Together, these results show that knockdown of NMDAR 2B subunit expression in the dorsal CA1 and cortex impairs visual-spatial discrimination learning in a training- and task order-specific manner.
The TUNL paradigm was developed to provide an automated measure of delayed non-matching-toposition (DNMTP) performance free of the effects of mediating behaviors in rodents. Variants of the task have been shown to be sensitive to loss of dorsal hippocampal function in rats and mice (Josey & Brigman, 2015; Kim et al., 2015; J. C. Talpos, S. M. McTighe, R. Dias, L.M. Saksida, & T. J. Bussey, 2010). The hippocampus has a well-established role in the formation and storage of episodic memories (Milner, Squire, & Kandel, 1998; Squire, Stark, & Clark, 2004), with distinct sub-regions implicated in efficient delayed non-matching tasks. While the role of the dentate gyrus (Clelland et al., 2009; Gilbert, Kesner, & Lee, 2001) and the CA3 (J. K. Leutgeb, Leutgeb, S., Moser, M. & Moser, E.I., 2007) are well established, the role of the CA1 in mediating visual-spatial discrimination learning is poorly understood. However, studies have suggested that CA1 hippocampal output may mediate spatial discrimination through its role in interpreting CA3 spatial mapping (S. Leutgeb & Leutgeb, 2007) and the timing of stimulus presentation (Hunsaker & Kesner, 2008).
Numerous studies have established a role for GluN2B-containing NMDARs in CA1 hippocampal plasticity. Pharmacological antagonism of GluN2B in slice results in a loss of LTP and LTD, as well as short-term potentiation in juvenile rats (France et al., 2017). Blockade of GluN2B activity in vivo in adult rodents following the induction of LTP can block its decay (Sachser et al., 2016) suggesting that GluN2B plays a critical role in updating memory traces. Indeed, GluN2B antagonism in the CA1 has been shown to impair hippocampal-dependent learning and memory tasks such as object recognition (Sachser et al., 2016) and contextual fear conditioning (Sun et al., 2016). Further supporting its role in plasticity, genetic overexpression of GluN2B in the CA1 enhances LTP in adult and aged mice, as well as adult rats (Cao et al., 2007; Tang et al., 1999; Wang et al., 2009). Although it should be noted that increased levels of GluN2B have been associated with pathological forgetting via the decay of established late LTP (l-LTP) (Hardt, Nader, & Wang, 2014). Utilizing the same genetic model described here, we have previously shown that loss of GluN2B in the CA1 impairs sub-saturating LTP, but not a saturating form (Brigman et al., 2010). GluN2BNULL mice also demonstrated robust impairments in traditional tasks of hippocampal function including Morris water maze and trace fear conditioning.
When tested on systematic variations of the TUNL paradigm, GluN2BNULL mice were initially impaired on the easiest training version of the task with a maximum separation and minimum delay, but could perform to a similar level as controls after sufficient training. When mice were tested on versions with fixed separations and increasing (but fixed within sessions) delays, GluN2BFLOX control mice performed consistently well, while GluN2BNULL mice performed significantly worse relative to controls. GluN2BNULL mice lack 2B subunit expression both within the CA1 region, and throughout the cortex which has been associated with increased perseveration during reversal and shifting behavior (Brigman et al., 2013; Dalton, Liya, Phillips, & Floresco, 2011; Thompson, Josey, Holmes, & Brigman, 2015). While not designed specifically as a measure of cortical function, TUNL task performance is likely affected by alterations in cortical function as it involves delay dependent and working memory. Along these lines, a large body of clinical evidence has shown that cortical function, as well as hippocampal, is involved in visual-spatial discrimination learning (Leal & Yassa, 2018). Rodent studies have shown that lesions of the medial prefrontal cortex alone can impair retention on DMTP that is delay dependent (Sloan, Good & Dunnett, 2006). Further, the perirhinal cortex has been posited to be involved in focus on content of the information rather than on processing of it (Kent, Hvoslef-Eide, Saksida, & Bussey, 2016). Not surprisingly, we found that during initial training on the easiest (4–5 spaces, 1 sec delay) variant, GluN2BNULL mice made significantly more correction trials during the first three sessions. However, this effect was absent by late phase of training and not seen in later problems and cohorts, suggesting that the GluN2BNULL mice chose a strategy of responding to familiar versus novel stimuli on choice trials early on, but learned the correct response rule with sufficient training. This is consistent with previous findings that global pharmacological antagonism of GluN2B does not alter TUNL performance when administered after stable performance of the task had already been established (Kumar, Olley, Steckler, & Talpos, 2015). Together, these data suggest that cortical and hippocampal CA1 GluN2B is required for initial acquisition of the task, and while all mice were sensitive to changes in separation regardless of delay, GluN2BNULL mice failed to benefit as greatly from extended training as control animals.
GluN2BNULL mice did not display significantly increased home-cage locomotor activity, although there were elevations during both dark phases. This is consistent with measures of latency to respond to stimuli or retrieve reward during TUNL testing, which did not differ between genotypes. Previous studies in mutant GluN2B mice have found no difference in activity during paradigms such as cued Morris water maze (Brigman et al., 2010) and instrumental responding (Brigman et al., 2013). Similarly, loss of GluN2B did not lead to changes in overall motor coordination or fine motor behavior (Thompson et al., 2015). This is in contrast to global pharmacological antagonism of GluN2B, which has previously been shown to increase reactions times for choice and reward collection behaviors, even in the absence of changes in accuracy (Kumar et al., 2015). These results underline that alterations in visual-spatial discrimination learning are likely not due to gross changes in activity resulting from alterations in NMDAR tone.
The current TUNL paradigm results are in line with previous studies indicating a role for CA1 in the temporal processing of episodic memory (Hunsaker, Lee, & Kesner, 2008), such as that required for efficient spatial discrimination. However, when tasked with identifying novel spatial stimuli on a challenge variant when both separation and delay were variable, all mice again demonstrated decreased performance by separation, but GluN2BNULL mice showed no loss in performance. As with systematic testing, this suggests that, with extensive task training, mutant animals may overcome impairments and reach control performance. Therefore, we examined whether a second cohort of mice tested on the variable challenge problem after only performing the easiest training variant (4–5 spaces, 1 sec. delay) would show altered performance. GluN2BNULL mice once again displayed decreased performance initially that normalized with training. GluN2BNULL mice now also displayed significantly reduced performance on all delays within the medium and minimum separations, which points to a possible role for the CA1 in delay-dependent visual-spatial discrimination, particularly when the spatial task is difficult. Further supporting this hypothesis, we previously found that targeted lesions of the dorsal hippocampus only disrupted performance in a delay-dependent manner when delays were variable (Josey & Brigman, 2015).
While the precise role that dorsal CA1 GluN2B-containing NMDARs plays in distinct stages of learning and performance on the TUNL task is still not known, the current data and previous results with non-regionally specific antagonism of GluN2B underlines the importance of both the cortex and CA1 in visual-spatial discrimination learning when delay periods are long and the task is difficult. Examination of hippocampal plasticity in GluN2B-knockout mice has shown an impairment in the ability of cells in the CA1 to produce LTP using sub-saturating stimulation, while LTP following saturating stimulation was unaltered (Brigman et al., 2010). Current theories posit that early LTP (e-LTP) has a much faster time course and is independent of the protein synthesis required for long lasting (hours to days) l-LTP (Lynch, 2004). Together with established deficits in sub-saturating LTP, early behavioral deficits in visual-spatial discrimination learning in GLuN2BNULL mice that are overcome with extensive training across days suggest that CA1 GluN2B containing NMDAR may play a critical role in e-LTP required for efficient trial-by-trial learning. GluN2B-dependent LTP in newborn DG neurons has been shown to be necessary both for LTP induction and spatial discrimination in highly similar, but not more distinct, contexts (Kheirbek, Tannenholz, & Hen, 2012). Here, we found that GluN2B loss in the cortex and CA1 impaired trial-by-trial learning early in training, or when problems were sufficiently challenging. Alternatively, session-by-session performance improved in mutant animals, suggesting that l-LTP processes may be spared. While further study is needed to examine how distinct phases of LTP underlie performance in the TUNL paradigm, these results support an important role of GluN2B in the CA1 in efficient learning.
In conclusion, we found that loss of GluN2B in the dorsal CA1 and cortex led to impairments in easy variants of the TUNL task that could be overcome with extensive training. However, challenging variants, with either fixed close separations and long delays or testing on the challenge variant with minimal training, revealed significant deficits in GluN2BNULL mice. Taken together, the current results demonstrate a unique role of GluN2B in the dorsal CA1 for visual-spatial discrimination learning that is sensitive to both the cognitive demands of each problem and the extent of the training period.
Highlights:
GluN2B in the cortex and CA1 is required for efficient learning of a touchscreen TUNL task.
GluN2B loss impairs early acquisition of an easy task variant, but deficits decrease with training.
Loss of GlluN2B impairs performance on systemic variations of both separation and delay.
Challenging variants are impaired in GluN2B mutants only when tested prior to extensive training.
Supports a unique role for the cortex and dorsal CA1 in spatial discrimination behavior.
Acknowledgments
We are very grateful to Dr. Benjamin Clark for his insightful comments and assistance with this manuscript. This work was supported by the NIAAA Intramural Research Program and grants 1K22AA020303–01, 1P50AA022534–01 and 5T32AA014127e13.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Kentridge RW, & Sembi S (1992). Lesions of the fornix but not the amygdala impair the acquisition of concurrent discriminations by rats. Behav Brain Res, 48(2), 103–112. [DOI] [PubMed] [Google Scholar]
- Brady ML, Allan AM, & Caldwell KK (2012). A limited access mouse model of prenatal alcohol exposure that produces long-lasting deficits in hippocampal-dependent learning and memory. Alcohol Clin Exp Res, 36(3), 457–466. doi: 10.1111/j.1530-0277.2011.01644.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Daut RA, Saksida L, Bussey TJ, Nakazawa K, & Holmes A (2015). Impaired discrimination learning in interneuronal NMDAR-GluN2B mutant mice. Neuroreport, 26(9), 489–494. doi: 10.1097/WNR.0000000000000373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Daut RA, Wright T, Gunduz-Cinar O, Graybeal C, Davis MI, Holmes A (2013). GluN2B in corticostriatal circuits governs choice learning and choice shifting. Nat Neurosci, 16(8), 1101–1110. doi: 10.1038/nn.3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK,Holmes A (2010). Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci, 30(13), 4590–4600. doi: 10.1523/JNEUROSCI.0640-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J,Saksida LM (2012). New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology, 62(3), 1191–1203. doi: 10.1016/j.neuropharm.2011.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Cui Z, Feng R, Tang YP, Qin Z, Mei B, & Tsien JZ (2007). Maintenance of superior learning and memory function in NR2B transgenic mice during ageing. Eur J Neurosci, 25(6), 1815–1822. doi: 10.1111/j.1460-9568.2007.05431.x [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD Jr., Fragniere A, Tyers P,Bussey TJ (2009). A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science, 325, 210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy SG, Brickley S, & Farrant M (2001). NMDA receptor subunits: diversity, development and disease. Current Opinion in Neurobiology, 11, 327–335. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Liya MM, Phillips AG, & Floresco SB (2011). Blockade of NMDA GluN2B receptors selectively impairs behavioral flexibility but not initial discrmination learning. Psychopharmacology, 216, 525–535. [DOI] [PubMed] [Google Scholar]
- France G, Fernandez-Fernandez D, Burnell ES, Irvine MW, Monaghan DT, Jane DE,Volianskis A (2017). Multiple roles of GluN2B-containing NMDA receptors in synaptic plasticity in juvenile hippocampus. Neuropharmacology, 112(Pt A), 76–83. doi: 10.1016/j.neuropharm.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, & Lee I (2001). Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus, 11(6), 626–636. doi: 10.1002/hipo.1077 [DOI] [PubMed] [Google Scholar]
- Hardt O, Nader K, & Wang YT (2014). GluA2-dependent AMPA receptor endocytosis and the decay of early and late long-term potentiation: possible mechanisms for forgetting of short- and long-term memories. Philos Trans R Soc Lond B Biol Sci, 369(1633), 20130141. doi: 10.1098/rstb.2013.0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland JG, & Cazakoff BN (2010). Effects of acute stress and GluN2B-containing NMDA receptor antagonism on object and object-place recognition memory. Neurobiol Learn Mem, 93(2), 261–267. doi: 10.1016/j.nlm.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, & Kesner RP (2008). Evaluating the differential roles of the dorsal dentate gyrus, dorsal CA3, and dorsal CA1 during a temporal ordering for spatial locations task. Hippocampus, 18(9), 955–964. doi: 10.1002/hipo.20455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Lee B, & Kesner RP (2008). Evaluating the temporal context of episodic memory: the role of CA3 and CA1. Behav Brain Res, 188(2), 310–315. doi: 10.1016/j.bbr.2007.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt DL, & Castillo PE (2012). Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr Opin Neurobiol, 22(3), 496–508. doi: 10.1016/j.conb.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josey M, & Brigman JL (2015). Loss of hippocampal function impairs pattern separation on a mouse touch-screenoperantparadigm. NeurobiolLearnMem,125,85–92. doi: 10.1016/j.nlm.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent BA, Hvoslef-Eide M, Saksida LM, & Bussey TJ (2016). The representational-hierarchical view of pattern separation: Not just hippocampus, not just space, not just memory? Neurobiol Learn Mem, 129, 99–106. doi: 10.1016/j.nlm.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Tannenholz L, & Hen R (2012). NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. J Neurosci, 32(25), 8696–8702. doi: 10.1523/JNEUROSCI.1692-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Romberg C, Hvoslef-Eide M, Oomen CA, Mar AC, Heath CJ,Saksida LM (2015). Trial-unique, delayed nonmatching-to-location (TUNL) touchscreen testing for mice: sensitivity to dorsal hippocampal dysfunction. Psychopharmacology. doi: 10.1007/s00213-015-4017-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G, Olley J, Steckler T, & Talpos J (2015). Dissociable effects of NR2A and NR2B NMDA receptor antagonism on cognitive flexibility but not pattern separation. Psychopharmacology, 232, 3991–4003. doi: 10.1007/s00213-015-4008-9 [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Kenji S, Manabe T, Takayama C, Katakura N, Kushiya E,…Mishina, M. (1996). Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor ε2 subunit mutant mice. Neuron, 16, 333–344. [DOI] [PubMed] [Google Scholar]
- Leal SL, & Yassa MA (2018). Integrating new findings and examining clinical applications of pattern separation. Nat Neurosci, 21(2), 163–173. doi: 10.1038/s41593-017-0065-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser M & Moser EI (2007). Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science, 315, 961–966. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, & Leutgeb JK (2007). Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn Mem, 14(11), 745–757. doi: 10.1101/lm.703907 [DOI] [PubMed] [Google Scholar]
- Lynch MA(2004).Long-termpotentiation and memory. Physiol Rev,84,87–136. doi: 10.1152/physrev.00014.2003 [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Carrow S, Place R, & Eichenbaum H (2013). Distinct hippocampal time cell sequences represent odor memories in immobilized rats. J Neurosci, 33(36), 14607–14616. doi: 10.1523/JNEUROSCI.1537-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt K, Saha M, Mishina M, Young JW, & Brigman JL (2014). Loss of GluN2A-containing NMDA receptors impairs extra-dimensional set-shifting. Genes Brain Behav, 13(7), 611–617. doi: 10.1111/gbb.12156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt K, Sigdel R, & Brigman JL (2017). Touch-screen visual reversal learning is mediated by value encoding and signal propagation in the orbitofrontal cortex. Neurobiol Learn Mem, 139, 179–188. doi: 10.1016/j.nlm.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B, Squire LR, & Kandel ER (1998). Cognitive neuroscience and the study of memory. Neuron, 20, 445–468. [DOI] [PubMed] [Google Scholar]
- Mu Y, & Gage FH (2011). Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Molecular Neurodegeneration, 6(85). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Hvoslef-Eide M, Heath CJ, Mar AC, Horner AE, Bussey TJ, & Saksida LM (2013). The touchscreen operant platform for testing working memory and pattern separation in rats and mice. Nat Protoc, 8(10), 2006–2021. doi: 10.1038/nprot.2013.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Hvoslef-Eide M, Kofink D, Preusser F, Mar AC, Saksida LM, & Bussey TJ (2015). A novel 2- and 3-choice touchscreen-based continuous trial-unique nonmatching-to-location task (cTUNL) sensitive to functional differences between dentate gyrus and CA3 subregions of the hippocampus. Psychopharmacology (Berl), 232(21–22), 3921–3933. doi: 10.1007/s00213-0154019-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachser RM, Santana F, Crestani AP, Lunardi P, Pedraza LK, Quillfeldt JA,Alvares Lde O (2016). Forgetting of long-term memory requires activation of NMDA receptors, L-type voltage-dependent Ca2+ channels, and calcineurin. Sci Rep, 6, 22771. doi: 10.1038/srep22771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro A (2013). Reassessing pattern separation in the dentate gyrus. Front Behav Neurosci, 7, 96. doi: 10.3389/fnbeh.2013.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE,Drew MR (2006). Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A, 103(46), 17501–17506. doi: 10.1073/pnas.0607207103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Hu C, Brigman JL, Zhu G, Hathaway HJ, & Prossnitz ER (2013). GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology, 154(11), 4136–4145. doi: 10.1210/en.2013-1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton OA, & Paulsen O (2014). GluN2A and GluN2B subunit-containing NMDA receptors in hippocampal plasticity. Philos Trans R Soc Lond B Biol Sci, 369(1633), 20130163. doi: 10.1098/rstb.2013.0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan HL, Dobrossy M, & Dunnett SB (2006). Hippocampal lesions impair performance on a conditional delayed matching and non-matching to position task in the rat. Behav Brain Res, 171(2), 240–250. doi: 10.1016/j.bbr.2006.03.042 [DOI] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, & Barnes CA (2011). A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci, 12 (10), 585–601. doi: 10.1038/nrn3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, & Clark RE (2004). The medial temporal lobe. Annu Rev Neurosci, 27, 279–306. doi: 10.1146/annurev.neuro.27.070203.144130 [DOI] [PubMed] [Google Scholar]
- Sun YY, Cai W, Yu J, Liu SS, Zhuo M, Li BM, & Zhang XH (2016). Surface expression of hippocampal NMDA GluN2B receptors regulated by fear conditioning determines its contribution to memory consolidation in adult rats. Sci Rep, 6, 30743. doi: 10.1038/srep30743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpos JC, McTighe SM, Dias R, Saksida LM, & Bussey TJ (2010). Trial-unique, delayed nonmatching-to-location (TUNL): a novel, highly hippocampus-dependent automated touchscreen test of location memory and pattern separation. Neurobiology of learning and memory, 94(3), 341–352. doi: 10.1016/j.nlm.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpos JC, McTighe SM, Dias R, Saksida LM, & Bussey TJ (2010). Trial-unique, delayed nonmatching-to-location (TUNL): a novel, highly hippocampus-dependent automated touchscreen test of location memory and pattern separation. Neurobiol Learn Mem, 94(3), 341352. doi: 10.1016/j.nlm.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Tsien JZ (1999). Genetic enhancement of learning and memory in mice. Nature, 401, 63–69. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Josey M, Holmes A, & Brigman JL (2015). Conditional loss of GluN2B in cortex and hippocampus impairs attentional set formation. Behav Neurosci, 129(2), 105–112. doi: 10.1037/bne0000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volianskis A, France G, Jensen MS, Bortolotto ZA, Jane DE, & Collingridge GL (2015). Longterm potentiation and the role of N-methyl-D-aspartate receptors. Brain Res, 1621, 5–16. doi: 10.1016/j.brainres.2015.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, & Williams MT (2014). Assessing spatial learning and memory in rodents. ILAR J, 55(2), 310–332. doi: 10.1093/ilar/ilu013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Cui Z, Zeng Q, Kuang H, Wang LP, Tsien JZ, & Cao X (2009). Genetic enhancement of memory and long-term potentiation but not CA1 long-term depression in NR2B transgenic rats. PLoS One, 4(10), e7486. doi: 10.1371/journal.pone.0007486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM (2004). The role of stimulus ambiguity and movement in spatial navigation: a multiple memory systems analysis of location discrimination. Neurobiol Learn Mem, 82(3), 216–229. doi: 10.1016/j.nlm.2004.05.004 [DOI] [PubMed] [Google Scholar]