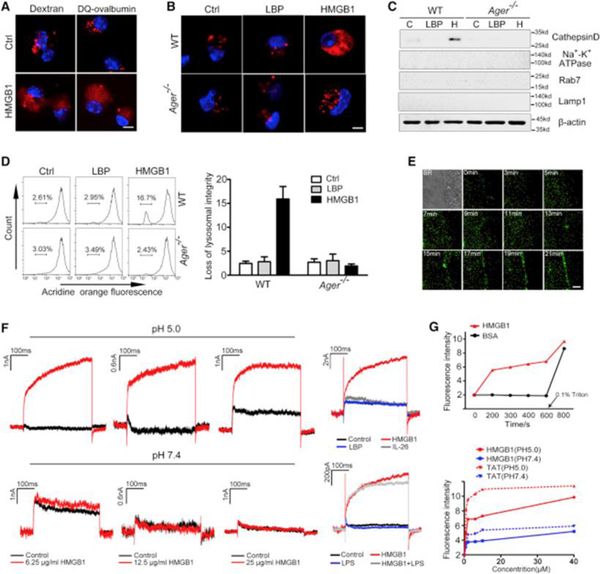
Figure 3. HMGB1 Destabilizes Lysosomal Membranes Leading to LPS Release into the Cytosol.
(A) Confocal microscopy of mouse peritoneal macrophages incubated with fluorescent dextran (red) or DQ ovalbumin (red) alone or together with HMGB1 (5 μg/mL) for 4 hr then fixed and stained with DAPI (blue). Scale bar: 10 μm.
(B) Confocal microscopy of WT or Ager−/− mouse peritoneal macrophages incubated with fluorescent dextran (red) alone or together with HMGB1 (5 μg/mL) or LBP (5 μg/mL) for 4 hr then fixed and stained with DAPI (blue). Scale bar: 10 μm.
(C) Immunoblot for Cathepsin D, Na+-K+ ATPase, Rab7, Lamp1, and β-actin in the cytosolic fraction from vehicle-treated (C) or HMGB1 (H, 5 μg/mL)- or LBP (5 μg/mL)-stimulated WT or Ager−/− mouse peritoneal macrophages.
(D) Flow cytometry of WT or Ager−/− mouse peritoneal macrophages stained with acridine orange and then treated with HMGB1 (5 μg/mL) or LBP (5 μg/mL) for 6 hr.
(E) Dynamic imaging of HMGB1 protein labeled with Alexa Fluor 488 (1 μg/mL) on living cell membranes. Scale bar: 2 μm.
(F) Whole-cell patch-clamp recording of HMGB1-induced inward current across the cytoplasmic membrane in proximity to the patch-clamp of HEK293 cells at neutral (pH = 7.4) or acidic (pH = 5.0) conditions.
(G) Fluorescent calcein dye was encapsulated into the liposomes, which were incubated with HMGB1, bovine serum albumin (BSA), or TAT at the indicated concentration for indicated time at neutral (pH = 7.4) or acidic pH (pH = 5.0). Liposome leakage was monitored by measuring calcein fluorescence intensity. Triton X-100 treatment was used to achieve 100% liposome leakage.
Graphs show the mean ± SD of technical replicates and are representative of three independent experiments. See also Figure S3.