Graphical abstract
Keywords: Pulsatile release, Sustained release, Etodolac, Bilayer tablet, Opadry, Surelease
Highlights
-
•
Bilayer tablet formulation of etodolac was formulated with a fast and a sustained release layers.
-
•
Compression of optimized fast and sustained release layers into a bilayer tablet.
-
•
Three successive coating layers of Opadry®, HPMC and Surelease® were applied on bilayer tablet.
-
•
In-vitro dissolution showed a lag time of 4 h followed by a prolonged release over 24 h.
-
•
Optimized formulation showed a prolonged anti inflammatory effect in rats.
Abstract
Repeated dose medication usually maximizes adverse effects, while sustained release systems did not offer a fast onset of action. Etodolac was formulated to enable pulsatile and sustained drug release, which was chronologically more suitable as an anti-inflammatory drug. Eudragit® RSPO, Eudragit® RLPO, and HPMC K15M were added in the sustained release layer and tried in different ratios. Croscarmellose sodium or sodium starch glycolate were used as superdisintegrants for the fast release layer offering the loading dose for rapid onset of drug action. Bilayer tablets were successively coated with Opadry®II, HPMC K4M and E5 (1:40), and Surelease®. All formulations complied with the Pharmacopeial standards for post-compression parameters. In-vitro release profile illustrated a lag-time of 4 h followed by a rapid loading dose release for 2 h. A prolonged steady state release with a t1/2 of 11 h lastly occurred. The coated bilayer tablet showed pulsatile and sustained release effects in rats. The licking time and swelling degree were tested and results demonstrated significant difference (P < 0.05) between the sustained anti-inflammatory action of formulation C1 compared to other groups. Therefore the new chronological design could provide a consistent drug release over 24 h with good protection against associated symptoms of gastric release.
Introduction
Rheumatoid arthritis is a chronic autoimmune disease that causes continuous articular devastation and bone deterioration. It is associated with chronic inflammation and tissue damage [1]. The night activation of the immune inflammatory reactions forces the symptoms to worsen in the early morning resulting in sleep disturbances related to quality and discontinuity [2]. Symptoms continue over the morning time and they are commonly represented by joint stiffness and functional disability [3]. Etodolac (ETD), a non-steroidal anti-inflammatory drug, is used to manage rheumatoid arthritis associated symptoms via inhibition of cyclooxygenase pathways and other inflammatory mediators [4]. ETD is a selective COX-2 inhibitor, which inhibits only cyclo-oxygenase-2 mediators. It causes less gastrointestinal complication compared to the majority of other NSAIDs [5].
Conventional delivery systems of ETD were found to engender stomach complications, such as nausea, epigastric pain, heartburn, and indigestion [6]. Delayed drug release formulation would be a suitable solution especially for chronic patients. In a patent assigned to Michelucci and Sherman [7], a sustained release dosage form of etodolac was provided in the form of matrix tablets with a release rate modifying agents. Although controlled release medication decreases the frequency of administration and diminishes the sleeping problems, yet the morning complications are not exterminated. Thus, a specialized drug delivery device is thought to be helpful in delivering a loading dose in the early morning and a maintenance dose over the day time. Therefore, researches were directed towards designing a bilayer tablet to include a fast release layer for rapid onset of action, beside a sustained release layer for drug level maintenance [8]. Nevertheless, the rapid drug release in the stomach prevents the success of the system, due to manifested side effects on gastric mucosa. Recently, another sigmoid release profile has attracted many workers interested in the field of pharmaceutical formulation, the so-called pulsatile drug delivery system. Multiple benefits could be acquired through the new design as the delivery device was capable of releasing the drug in a controlled programmable strategy after a precisely calculated lag phase [9]. Different formulation approaches could be applied with the new design, either single or multiunit systems supplied with controlled release coating materials. Multi-coating of tablets with time dependent polymers providing a lag-time prior to drug release initiation could attain the goal for pulsatile release [10].
Fast release layer formulations were furnished with superdisintegrants like Sodium starch glycolate and croscarmellose sodium to ensure expeditious drug release due to their ability to fragmentize the layer in few seconds [11]. Sustained release layer formulations depending on swelling mechanism could be simply manufactured through the addition of synthetic and polysynthetic polymers. Eudragit® RSPO and Eudragit® RLPO are pH independent polymethacrylate polymers containing quaternary ammonium groups. These polymers are characterized by their capability in sustaining the drug release rate [12]. HPMC is a semisynthetic polymer that is widely used in the pharmaceutical industry. It has been able to sustain the drug release through swelling and gelation when it gets in contact with dissolution fluids [13]. The current work combines formulation, evaluation and optimization of ETD coated bilayer tablets offering a combination of fast release and sustained release doses with a stomach protection from ETD adverse effects. The fast release layer provides the initial dose rapidly away from the stomach after a lag time elapse (pulsatile drug delivery), whereas the sustained release layer discharges its dose in a slow rate. Successive deposition of OpadryII®, HPMC E5 and Surelease® would result in delayed drug delivery.
Material and methods
Material
Etodolac (ETD) and Avicel PH-101 were kindly gifted from Global NAPI (GNP) (Cairo, Egypt), Croscarmellose sodium (CCNa) and Sodium starch glycolate (SSG) are gifts from Pharco Pharmaceuticals (Alexandria, Egypt), three grades of HPMC (E5, K4M and K15M), OpadryII® and Surelease® were gifted from Colorcon Limited (Kent, UK), PEG 6000 was purchased from Research Lab Fine-Chem Industries (Mumbai, India), Eudragit® RSPO and Eudragit® RLPO were gifted from Evonik Industries (North Rhine-Westphalia, Germany) and maize starch and magnesium stearate were purchased from El-Nasr Pharmaceutical Company (Cairo, Egypt).
Determination of equilibrium solubility of ETD in water
An excess amount of ETD was added in a plastic cap screw glass vial containing 10 mL of water. The vial was placed in an incubator, set to shake at 75 rpm at 37 ± 0.5 °C for 24 h, then the vial was allowed to rest for another 24 h at the same temperature, then the content was filtered, diluted appropriately and measured using a UV–Visible spectrophotometer (Shimadzu UV 1800 PC, Shimadzu, Kyoto, Japan) at 278 nm which corresponds to the λmax of the ETD.
Determination of equilibrium solubility of ETD-PEG 6000 in water
ETD was mixed with PEG 6000 in a ratio 1:1 by two methods, physical mixing and solid dispersion. The first method was performed by physical blending of ETD and PEG 6000. The second one was performed using the solvent evaporation technique. In such trial, ETD was dissolved in the minimum amount of methyl alcohol. Equal amount of PEG 6000 was added to the methanolic solution of the drug. The solution was placed in the flask of the rotary evaporator (Eyela Rotary Evaporator SB-1000, Eyela Co., Tokyo, Japan). The solvent was removed under reduced pressure at 50 °C and dried under vacuum at room temperature for 5 h. The solid sample was collected at the end of the test. Finally, the saturated solubility of ETD in both trials was determined and compared to the pure drug solubility in water [14].
Evaluation of pre-compression parameters
According to USP specifications, the angle of repose of the powdered mixture was determined by fixed funnel and free-standing cone method. A funnel was fixed in a certain position where a glass slab was placed 2 cm beneath its lower tip. Powder mixture was slowly and carefully poured through the funnel until the apex of the conical powder pile touched the funnel’s lower tip.
Bulk density (ρbulk) expressed in g/mL was determined by measuring the volume of a known weight (m) of a powder sample into a graduated cylinder. The apparent volume (V0) was carefully read to the nearest graduated unit, then the bulk density was calculated. The cylinder was then placed in Tapped Density Tester (Copley® Scientific Limited, Nottingham, United Kingdom) and was tapped for 100 times, then the volume was recorded as the final tapped volume (Vf). Then the tapped density was calculated [15].
The compressibility index is an indication of both powder compressibility as well as flow properties. Carr’s index and Hausner’s ratio were calculated [15].
Preparation of single layers of tablets
Preparation of the fast release layer
Fast release formulations were categorized into three groups. Each group contained the same amount of ETD, polymers and excipients differing only in the added type of disintegrant. The first group included SSG (F1), the second contained CCNa (F2) and the third one, maize starch (F3). Ingredients were separately weighed using a sensitive balance Sartorius AG, Göttingen, Germany). First, 200 mg from each of ETD and PEG 6000 were mixed using solid dispersion technique as previously mentioned. CCNa, SSG or starch and Avicel PH-101 were mixed with the premix in a geometric manner using a mortar and a pestle for 15 min. Prior to compression, magnesium stearate was added to the mixture and remixed. Finally, 600 mg of the mixture was filled in the die cavity of a single punch tablet machine (Royal Artist, Mumbai, India) equipped with 12 mm flat faced punches where it was compressed.
Preparation of the sustained release layer
Different formulations containing the same amount of ETD but different in polymers’ ratios were prepared. These formulations were divided into four sets. The first contained Eudragit® RSPO only (S1). The second contained Eudragit® RLPO only (S2). The third set combined the first two sets through the incorporation of both grades of Eudragit® in different ratios (S3 – S5). The last set was characterized by the presence of HPMC polymer in different ratios with Eudragit® RSPO and Eudragit® RLPO (S6 and S7). Sustained release tablet formulations were prepared as follows: PEG 6000 was added to ETD to increase its solubility using the solid dispersion method. Avicel PH-101, Eudragit® RSPO, Eudragit® RLPO and/or HPMC K15M were added to the previously prepared mixture and mixed for 15 min. Magnesium stearate was added to the premix prior to the compression and mixing continued for another 5 min. Then 1600 mg of the final mixture was placed in the die cavity of a single punch tablet press machine, equipped with 12 mm flat faced punches and finally compressed.
Preparation of bilayer tablets
Bilayer tablets were prepared through combination of the optimized fast release formulation with that of the sustained release one. The ingredients of the optimum sustained release formulation were mixed and placed in the die cavity of single-press tablet machine equipped with 12 mm biconcave punches. The powder was compressed with a low force of compression. The fast release powder mixture was placed above the intact sustained release layer and compressed with a higher force resulting in the formation of bilayer tablet. The composition of different formulations is shown in Table 1.
Table 1.
Composition of fast release, sustained release and bilayer tablet formulations.
| Composition | Weight of fast release formulations (mg) |
Weight of sustained release formulations (mg) |
Optimized Bilayer Formulation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | S1 | S2 | S3 | S4 | S5 | S6 | S7 | B1* | |
| ETD | 200 | 200 | 200 | 400 | 400 | 400 | 400 | 400 | 400 | 400 | 600 200F + 400S |
| PEG 6000 | 200 | 200 | 200 | 400 | 400 | 400 | 400 | 400 | 400 | 400 | 600 200F + 400S |
| SSG | 30 | – | – | – | – | – | – | – | – | – | 30S |
| CCNa | – | 10 | – | – | – | – | – | – | – | – | – |
| Starch | – | – | 75 | – | – | – | – | – | – | – | – |
| Eudragit® RSPO | – | – | – | 400 | – | 400 | 200 | 200 | 200 | 400 | 400S |
| Eudragit® RLPO | – | – | – | – | 400 | 200 | 200 | 400 | 200 | – | – |
| HPMC K15M | – | – | – | – | – | – | – | – | 160 | 160 | 160S |
| Avicel PH-101 | 165 | 185 | 120 | 390 | 390 | 190 | 390 | 190 | 230 | 230 | 395 165F + 230S |
| Magnesium stearate | 5 | 5 | 5 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 15 5F + 10S |
| Total | 600 | 600 | 600 | 1600 | 1600 | 1600 | 1600 | 1600 | 1600 | 1600 | 2200 |
Weights of ingredients in fast release layer were followed by “F”, while those in sustained release layer were given “S”.
Coating of bilayer tablets
Isolation layer coating
Sealing of the formulated bilayer tablets was achieved through coating with 10% (w/v) aqueous solution of OpadryII®. Prior to coating, OpadryII® solution was supplied with 50 mg of HPMC K4M to avoid early disintegration of tablets. The solution was continuously stirred with a magnetic stirrer (MSH-20A, Wise Stir®, Seoul, Republic of Korea) to prevent precipitation. A batch of the selected bilayer tablet was placed in a coating pan (PharmaCoating Pan CP-9, Hainburg, Germany) running at 8 rpm, where 100 mL of solution was constantly sprayed at a rate of 2 mL/min. During coating, inlet air was supplied from a dryer (Remington Compact 1800, Guangdong, China) whose temperature was adjusted to nearly 40 °C. Coated tablets were transferred to an oven (CO-150, HumanLab Instruments Co., Gyeonggi, Republic of Korea), adjusted at 50 °C for 4 h to ensure complete drying.
Swelling layer coating
Low viscosity grade HPMC E5 was dispersed in purified water to achieve a concentration of 8% (w/v) in addition to 200 mg of HPMC K4M. The prepared solutions were heated to 80 °C using a hot plate. The previously coated tablets (with isolation layer) were placed again in the coating pan. The rotation rate was 10 rpm. The coating solution was sprayed at a rate of 2 mL/min and the temperature of the inlet air was set at 40 °C. Tablets were then dried in an oven at 50 °C for 4 h.
Rupturable layer coating
The provided solution of Surelease® was diluted by purified water to achieve a concentration of 10% (w/v) of ethyl cellulose polymer. The solution was sprayed (onto tablets previously coated with the two successive layers) in the coating pan running with the same specifications as the previous steps. Finally, the tablets were dried in the oven for 4 h.
Evaluation of post-compression parameters
Fast release formulations, sustained release formulations and bilayer tablets were subjected to post-compression tests including the uniformity of thickness (n = 20), diameter (n = 20), weight (n = 20), and disintegration time (n = 6). It has to be noted that the disintegration of bilayer tablets were monitored in simulated gastric fluid and photographs were captured at 5, 10, and 15 min after immersion in the medium. For clarity and distinction of disintegration phases, the bilayer tablets under test were colored with orange and yellow colors for the fast and sustained layers respectively. In addition, the mechanical strength of tablets (n = 10) was determined by automated hardness tester (Dr Schleuniger Pharmatron AG CH-4500, Westborough, USA). Tablets (n = 20) were placed in a friabilator (Friability tester FRV2000, Copley® Scientific Limited, Nottingham, United Kingdom) to assess the percent weight loss accounting for their friability. The coated tablets were evaluated using the same tests with exception of the uniformity of thickness and diameter.
In-vitro drug release study
The dissolution testing for each of the fast release formulations, sustained release formulations and bilayer tablets was carried out according to the USP monograph using USP dissolution apparatus type I (SR8Plus, Hanson Research, California, USA) at 37 ± 0.5 °C and a stirring rate of 100 rpm. The tablet was placed in 1000 mL of 0.1 N HCl for the first 2 h then it was transferred to pH 6.8 phosphate buffer till the end of the experiment. At different time intervals, 5 mL samples were withdrawn at 5, 10, and 15 min in case of fast release formulations, and at 0.25, 0.5, 1, 2, 4, 6, 8, 12, and 24 h in case of sustained release formulations and bilayer tablet formulations. All samples were filtered through a cellulose acetate filter (pore size is 0.45 μm), diluted if needed and analyzed using UV–Visible spectrophotometer at 278 nm. Each withdrawn sample was compensated with 5 mL of the same fresh medium. All experiments were carried out in triplicates.
Kinetic analysis of release data
Data obtained from release experiments were treated statistically according to linear regression analysis. Data were then fitted to zero order, first order, and Higuchi model. Kinetic data were computed from the order of the best fit.
Physico-chemical characterization of the formulation
Differential scanning calorimetry (DSC)
ETD thermograms were performed using a differential calorimeter (Perkin-Elmer, Waltham, USA) to determine the DSC thermal traces. Samples of ETD, HPMC, Eudragit® RSPO, Eudragit® RLPO, PEG 6000 and physical mixture of the ingredients were weighed and placed in a standard aluminum pan. The instrument was calibrated with indium, dry nitrogen was used as a carrier gas with a flow rate of 20 mL/min and a scan speed of 10 °C /min up to 300 °C was employed. The weight of each sample was 5–10 mg. The main transition temperature (Tc) was determined as the onset temperature of the highest peak. Enthalpy values (ΔHm) were automatically calculated from the area under the main transition peak. The heat flow was measured for all samples [16].
Fourier transform infrared (FT-IR) spectroscopy
FT-IR spectra were obtained for the pure ETD, HPMC, Eudragit® RSPO, Eudragit® RLPO, Avicel PH-101, PEG 6000, CCNa, SSG, magnesium stearate and physical mixture of the ingredients of the optimized formula in the range of 4000–500 cm−1. Each sample was placed in the light path of sample cell of FT-IR spectrophotometer (Cary 630, Agilent Technologies, Danbury, USA) and the spectrum was recorded.
Study of surface topography
In order to illustrate the difference in surface topography of successive layers, the optimized bilayer tablet was transversely sectioned and examined under the scanning electron microscope. Sections were positioned on a sample holder (JEOL JSM 5300 Scanning Microscope, Tokyo, Japan). Samples were gold coated for 30 min (JEOL JFC 1100e Sputtering device, Tokyo, Japan) and images were obtained at (25 KeV × 350, 30 KeV × 100, 30 KeV × 500).
In-vivo anti-inflammatory activity
Formalin-induced edema in rat hind paw test was performed to evaluate the anti-inflammatory effect of the optimized ETD bilayer tablet [17]. This test is responsive for the anti-inflammatory activity of ETD in rats. The hind paw licking time and the swelling degree were the factors assessing the anti-inflammatory effect.
Preparing and housing of animals
Healthy albino rats of both sexes with average weight about 254 ± 16 g were divided into three groups (six rats in each), A, B and C. Each group consisted of six rats. All the groups were housed in the same place under the same circumstances of temperature, humidity and light. The animal experiment in this study was conducted according to the guidelines of the Ethical Committee for care and use of laboratory animals established by Faculty of Pharmacy and Drug Manufacturing, Pharos University in Alexandria and approved by the Research Ethics Committee of Pharos University in Alexandria (Approval No.: 25-18), which established the regulatory rules for animal research ethics on accordance to the National Institute of health guide for the care and use of laboratory animals. All rats were served the same type of food. They were fasted 12 h before testing, but got free access of water.
Preparation of rats’ formulations
The prepared bilayer tablets were not suitable for testing in rats due to their large dimensions and weights. Such tablets would result in rats shocking and death. Therefore, dose adjustment was done to suit rats’ body weight in order to be administered safely. The optimized formulation was downscaled according to Eq. (1) suggested by Osman and Atya [18]. Tablets were coated using the parameters and ingredients previously mentioned.
| (1) |
Treatment and evaluation
Before testing, the circumference of the ankle joint of the right hind paw was measured and titled as “zero-time circumference”. The tablets containing 14 mg of ETD were given orally to the rats. Thirty min later, 30 µL of 5% formalin in 0.9% saline was injected into the dorsal surface of the rats’ right hind paw with a micro-syringe equipped with 27-gauge needle. To perform the test, each group received the treatment according to the following sequence; the control group (A) remained without treatment and this was used as a reference. The control ETD group (B) received conventional ETD tablet (ETD, PEG 6000 and Avicel only), and group (C) received the optimized ETD formulation. Each rat was immediately returned to a Plexiglas® observation chamber. The degree of pain intensity was evaluated as the total time the animal spent licking the inflamed paw. This was visually monitored using a digital stopwatch. The licking times observed were converted into percentage maximum possible effect (%MPE) which could be calculated from equation (2) [17].
| (2) |
Swelling degree of the right hind paw of the three groups was calculated as an assessment of the degree of inflammation from equation (3). Results were subjected to one-way ANOVA test where P-value of ≤0.05 was considered statistically significant [19].
| (3) |
Where C1, circumference of right hind paw before drug administration and C2, circumference of the right hind paw after drug administration.
Results and discussion
Determination of equilibrium solubility of ETD in water
ETD is practically insoluble in water [20]. Results of solubility study in water showed that maximum solubility of pure ETD powder was 27 mg/L. The incorporation of PEG 6000 to the drug showed a great influence on its solubility. Physical mixture of ETD and PEG 6000 led to an increase in the drug solubility into 142 mg/L (≈5.3 folds). Whereas, the last trial performed using the solid dispersion technique by the solvent evaporation method shifted the drug solubility by nearly 18 folds (500 mg/L) compared to ETD alone [14].
Evaluation of pre-compression parameters
The powdered mixture of both fast release and sustained release formulations showed accepted flowability characteristics. Their bulk densities varied from 0.253 to 0.444 g/mL, while their tapped densities ranged from 0.282 to 0.546 g/mL. Carr’s index of the formulations varied between 10.30% and 21.68%, accordingly, Hausner’s ratio ranged from 1.115 to 1.277. The flowability of all formulations ranged from passable to good. Carr’s index and Hausner’s ratio should not exceed 25% and 1.34, respectively.
Evaluation of post-compression parameters
Fast release formulations
The physical testing of fast release formulations showed that the average weight of tablets was nearly equal. Results of the weight uniformity test for the three batches complied with USP requirements. The percentage weight loss for each of the three batches was less than 1%. Average value for hardness was in the range of 41.67–43 N. Formulations containing SSG and CCNa were placed in disintegration apparatus (Copley® Scientific Limited, Nottingham, United Kingdom), and were found to consume less than 2 min to achieve complete disintegration, which is shorter than the formulation containing starch.
Sustained release formulations
Tablets from different batches were evaluated to ensure their weight uniformity. Results show that all batches fell in the acceptable pharmacopeial limit data not shown. The average diameter was almost the same. Tablets showed similar values for hardness, and none of the batches exceeded the maximum acceptable weight loss during friability testing. The post-compression data of the optimized fast and sustained release formulations are shown in Table 2.
Table 2.
Post compression parameters of fast and sustained release formulations.
| Formulation Code | Thickness (mm) | Diameter (mm) | Weight (mg) | Hardness (N) | Friabilitya | Disintegration time (min) |
|---|---|---|---|---|---|---|
| F1 | 4.59 ± 0.094 | 12.215 ± 0.067 | 599.35 ± 2.417 | 41.67 ± 1.033 | 0.348% | 1.52 ± 0.24 |
| F2 | 4.64 ± 0.094 | 12.22 ± 0.052 | 599.235 ± 2.163 | 43.33 ± 1.966 | 0.386% | 2.03 ± 0.54 |
| F3 | 6.325 ± 0.224 | 12.235 ± 0.049 | 598.995 ± 3.019 | 43.00 ± 2.449 | 0.529% | 4.96 ± 0.65 |
| S1 | 7.075 ± 0.102 | 12.19 ± 0.085 | 1595.97 ± 8.646 | 46.17 ± 2.317 | 0.587% | >120 |
| S2 | 7.105 ± 0.115 | 12.22 ± 0.083 | 1592.79 ± 5.948 | 46.50 ± 2.168 | 0.524% | >120 |
| S3 | 8.445 ± 0.076 | 12.23 ± 0.047 | 595.345 ± 6.435 | 47.67 ± 2.338 | 0.708% | >120 |
| S4 | 7.13 ± 0.130 | 12.235 ± 0.059 | 1594.31 ± 5.660 | 46.17 ± 2.483 | 0.421% | >120 |
| S5 | 8.50 ± 0.134 | 12.23 ± 0.047 | 1596.87 ± 3.978 | 46.33 ± 2.160 | 0.831% | >120 |
| S6 | 9.485 ± 0.160 | 12.235 ± 0.059 | 1595.48 ± 6.629 | 46.50 ± 2.074 | 0.882% | >120 |
| S7 | 9.580 ± 0.199 | 12.210 ± 0.055 | 1594.72 ± 4.230 | 46.33 ± 2.160 | 0.663% | >120 |
Percentage weight loss.
In-vitro drug release study
Drug release from fast release formulations
In-vitro release profiles of ETD from fast release tablet formulations was studied in 1000 mL 0.1 N HCl (pH 1.2) for 15 min. The aim of this test was to compare between three disintegrants according to their types in order to get a rapid release as mentioned in Table 1. The role of disintegrants was to promote moisture penetration which initiated disintegration and subsequently facilitated drug release from tablets matrices. Fig. 1 shows that all formulations experience fast release except for the one having ordinary starch as disintegrant (F3) that shows slower release than those containing superdisintegrants (F1 and F2).
Fig. 1.
In-vitro release profiles of ETD from different fast release formulations containing different types of disintegrants in simulated gastric fluid (pH 1.2).
F1 showed the highest release among tested superdisintegrants after 15 min. The composition and nature of SSG was probably the key for its disintegration role. The presence of carboxymethyl groups in this polymer caused disruption of hydrogen bonding within the structure. This permitted the penetration of water into the molecule, then the polymer became water soluble [11]. Hence, the disintegration mechanism by which SSG fragmented the tablet was mainly through rapid absorption of water followed by swelling leading to an enormous increase in polymer granules volume which resulted in rapid and uniform disintegration. While the natural starches (maize starch) swelled in water to the extent of 10 – 20%, modified starches (SSG) increased in volume by 200 – 300% in water [11]. Tablets formulated with these superdisintegrants were thus disintegrated in less than two min. CCNa incorporated in F2 is a cross-linked polymer of carboxymethyl cellulose. This cross-linking rendered it insoluble, highly hydrophilic, with excellent swelling properties and a unique fibrous nature. This gave the polymer excellent water wicking capabilities [21]. In addition, CCNa swelled rapidly in water without much gelling. Thus, CCNa performed its disintegration role through the capillary action when it swelled and reduced the physical binding forces between particles [21].
Drug release from sustained release formulations
Eudragit® RSPO, Eudragit® RLPO and HPMC K15M were shown to effectively prolong the drug release. Addition of single grade of Eudragit® was tried in two formulations (Table 1). Both Eudragit® RSPO and Eudragit® RLPO are pH-independent polymers which are impermeable to water. These polymers contain quaternary ammonium groups in their chemical structure. The solubilization of these groups led to the formation of pores in the tablet matrix allowing water to enter by diffusion [22]. Fig. 2 shows the in-vitro release profile of different sustained release formulations. Eudragit® RSPO showed better sustaining capability than Eudragit® RLPO. This was probably because Eudragit® RSPO had a less proportion of quaternary ammonium groups in its structure which was responsible for low water permeability and swellability [23]. When the two polymers were mixed together, Eudragit® RSPO was found to retard the drug release to reach 12 h, while increasing amount of Eudragit® RLPO fastened the drug release due to its more hydrophilic character which assisted the system hydration and increased the water absorption [24] and tablet erosion accordingly. Incorporation of HPMC K15M in S6 and S7 provided a greater effect in sustaining the drug release than Eudragit® polymers alone or in combination. HPMC is a water soluble cellulose derivative, but it forms insoluble matrix when combined with Eudragit® RLPO and RSPO. HPMC formed a firm gel layer along with Eudragit® RLPO and RSPO and helped in formation of pores on the tablet surface. Also because of its tendency to mask the quaternary ammonium groups of Eudragit® RLPO and RSPO to some extent, it modified the drug release rate from the matrix [25].
Fig. 2.
In-vitro release profiles of ETD from different sustained release formulations containing Eudragit® RSPO, Eudragit® RLPO and/or HPMC in simulated gastric fluid (pH 1.2) for 2 h then in simulated intestinal fluid (pH 6.8).
Drug release from bilayer tablet formulations
The bilayer tablets consisted of two distinct parts. The fast release layer was formulated in order to achieve rapid drug release after administration. It contained SSG as a superdisintegrant, which caused the first layer to disintegrate rapidly releasing the necessary loading dose of the drug. When the concentration started to diminish as a result of drug exhaustion, a maintenance dose was provided by the sustained release layer. Such layer was formulated using two polymers (Eudragit® RSPO and HPMC K15M) to sustain the drug release and maintain its rate constant over prolonged period of time. In-vitro release profile of ETD was studied by placing the bilayer tablet in simulated gastric fluid (pH 1.2) for 2 h followed by changing the medium into simulated intestinal fluid (pH 6.8). During the first 2 h, the particles of superdisintegrant incorporated in the fast release layer started to absorb water from the surrounding medium resulting in swelling of these particles and rapid rupture in the layer leading to disintegration. The disintegration resulted in a complete drug release of the fast release layer within 15 min (Fig. 3). The first five min caused the fast release layer to swell and particles were dispersed into the medium as shown in Fig. 3a. Five min later, the dimensions of that layer increased due to the increase in the swelling degree as shown in Fig. 3b. Disintegration was completed after 15 min. This was determined by the disappearance of the orange color as observed in Fig. 3c. The bilayer tablet was then transformed into a single layer.
Fig. 3.
Disintegration levels of the fast release layer of the bilayer tablet in simulated gastric fluid (pH 1.2) after (a) 5 mins, (b) 10 mins and (c) 15 mins.
The sustained release layer of B1 formulation required up to 24 h to release the drug completely. The same tested formulation went through coating processes to be transformed into a coated bilayer tablet (C1). The Surelease® incorporated in the outermost layer is a polymer dispersion made of EC (18.5%). It acts as the rate controlling factor of drug release. The purpose of using this rupturable layer was to decrease the release rate of ETD. Once the in-vitro testing started, the dissolution medium crossed the Surelease® layer through tiny cracks [26]. The entrance of the fluid to the inside of the layer caused its rupture.
The second layer made of HPMC E5 and K4M (40:1) was thus exposed to the dissolution medium, the fluid crossed HPMC slowly through the small pores found on the surface, generating pressure gradually on the coat. Eventually, this thorough diffusion led to swelling of the polymer layer forming a high viscosity gel matrix. The presence of HPMC K4M in the swellable layer led to an increased hydration, which in turns resulted in higher swelling degree and erosion [27]. The innermost coating layer adjacent to the bilayer tablet was made of OpadryII®. It is a water soluble, pH independent polymer consisting of polyvinyl alcohol, titanium dioxide, talc and PEG 3350. The main purpose of using OpadryII® was to reinforce the bilayer tablet so that it could withstand the following coating processes [28]. In addition, it provided a protective film over the tablet to prevent any chemical degradation of the tablet that might be caused by the successive coating solutions. It also guaranteed glossy and smooth surface, ensuring continuous film formation for the successive coating layers. Its absence might affect the final coated product behavior and led to unexpected drug release profile due to probable wrinkles on the surface.
The swelling and erosion of the three coat layers consumed around 4 h. During this period, the drug release was nil as the dissolution fluid was still on its way to penetrate the tablet. This provided an intended lag time corresponding to the time necessary for the evacuation of the formulation from the stomach. In-vitro release profile of bilayer tablets is shown in Fig. 4.
Fig. 4.
In-vitro release profiles of ETD from bilayer tablet before and after coating in simulated gastric fluid (pH 1.2) for 2 h then in simulated intestinal fluid (pH 6.8).
Kinetic analysis of release data
The in-vitro drug release data for fast and sustained release formulations were analyzed using the mathematical models: zero order kinetics, first order kinetics and Higuchi model. F1 and S7 were chosen as the optimized formulations according to the obtained kinetic data shown in Table 3. Subsequently, the constituents of F1 and S7 were combined in bilayer tablets. Results showed that the optimized bilayer tablet formulation (B1) obeyed Higuchi model (r2 = 0.9571) with a t1/2 of 4.3 h, while the optimized coated bilayer tablet (C1) followed zero order kinetic model (r2 = 0.999) with a t1/2 = 11.14 h where the first time point considered was at 6 h after attainment of steady state. This assures the controlled drug release from C1 formulation.
Table 3.
Linear regression and kinetic analysis of release data of fast, sustained, bilayer and coated formulations.
| Formulation code | Linear regression analysis (r2)* |
Kinetic analysis |
|||
|---|---|---|---|---|---|
| Zero order | First order | Higuchi | K** | t1/2 (h) | |
| F1 | 0.8487 | 0.9762 | 0.9408 | 13.736 | 0.05 |
| F2 | 0.8503 | 0.9638 | 0.9384 | 7.269 | 0.095 |
| F3 | 0.9214 | 0.9639 | 0.9609 | 4.562 | 0.152 |
| S1 | 0.9420 | 0.9055 | 0.9967 | 27.855 | 3.222 |
| S2 | 0.9244 | 0.9292 | 0.9867 | 34.542 | 2.095 |
| S3 | 0.9115 | 0.9544 | 0.9907 | 29.192 | 2.934 |
| S4 | 0.8832 | 0.9823 | 0.9699 | 0.441 | 1.573 |
| S5 | 0.8674 | 0.9780 | 0.9617 | 0.475 | 1.459 |
| S6 | 0.8780 | 0.9782 | 0.9905 | 20.766 | 5.797 |
| S7 | 0.9095 | 0.9574 | 0.9973 | 20.046 | 6.221 |
| B1 | 0.8965 | 0.9267 | 0.9537 | 24.185 | 4.274 |
| C1 | 0.9990 | 0.9746 | 0.9971 | 4.488 | 11.14 |
The underlined data correspond to the order of the best fit.
K in h−1 for F1–F3, S4, S5 and in mg h−1/2 for S1-S3, S6, S7, B1, C1.
Physico-chemical characterization of the formulation
Differential scanning calorimetry (DSC)
DSC is used in pharmaceutical industry to allow evaluation of possible incompatibilities between different components blended in the formulation according to the appearance, shift and disappearance of peaks in the corresponding enthalpies. DSC curves (shown in Fig. 5A) were used to determine the compatibility of ETD with various added excipients. The DSC curve of crystalline anhydrous ETD showed a sharp endothermic peak at 150.45 °C corresponding to its melting point and onset of 148.61 °C with a melting enthalpy of 125 J g−1 [14]. HPMC showed wide endothermic peak at 64.6 °C due to the polymer dehydration [29]. The curve related to PEG 6000 displayed an endothermic peak at 68.4 °C corresponding to its melting temperature [14]. Moreover, both Eudragit RSPO and Eudragit RLPO showed nearly flat thermal curve as mentioned in the literature indicating their amorphous nature [30]. Eudragit RSPO showed a weak peak at 64.47 °C with a melting enthalpy of 2.44 J g−1, while Eudragit RLPO showed wider peak at 65.79 °C. Fig. 5A, Fig. 5B, Fig. 5C, Fig. 6 shows the DSC profile for the physical mixture of the previous components. The display shows only one peak characteristic to the melting point of PEG 6000 which probably overlaps the nearby peaks of Eudragit and HPMC. The disappearance of endothermic peak of ETD was due to the complete solubility of ETD in melted PEG 6000 at temperature lower than the drug melting point [14].
Fig. 5A.
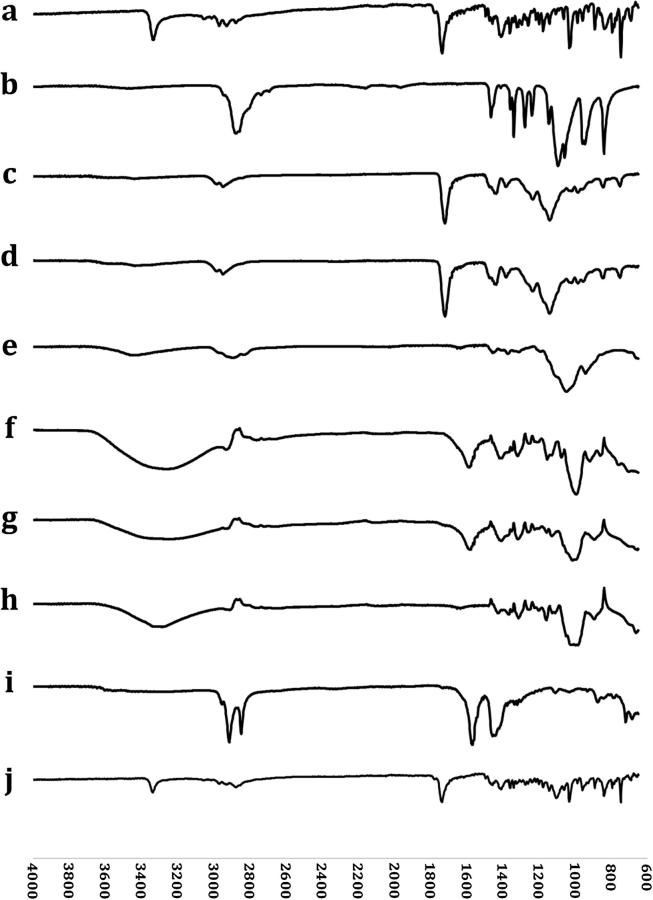
FT-IR spectra of (a) ETD, (b) PEG 6000, (c) Eudragit® RSPO, (d) Eudragit® RLPO, (e) HPMC, (f) Avicel PH-101, (g) SSG, (h) CCNa, (i) Magnesium stearate and (j) powder mixture.
Fig. 5B.
DSC curves of (a) ETD, (b) HPMC, (c) PEG 6000, (d) Eudragit® RSPO, (e) Eudragit® RLPO, and (f) Mixture of the optimized formulation.
Fig. 5C.
SEM photomicrographs of (a) bilayer tablet, (b) fast release layer, (c) and (d) sustained release layer.
Fig. 6.
Swelling degrees in rats groups after inflammation induction.
Fourier transform infrared (FT-IR) spectroscopy
The results of FT-IR spectra of the ingredients used in the formulations are illustrated in Fig. 5B, in which ETD shows peaks at 2929 and 2971 cm−1 due to presence of stretching vibration (C—H) corresponding to 2930 and 3000 cm−1 in the FT-IR spectrum. The next peak was at 1736 cm−1 due to the presence of the stretching vibration of alkene (C C). The (C—H—C) scissor bond was determined at 1362 cm−1, while the angle bending of the bond (C—C—H) resulted in peaks at 1143 and 1200 cm−1. The FT-IR spectrum showed peaks at 747, 795, and 839 cm−1 due to the twisting vibrations out of the plane ring. This observation is in agreement with that reported by Dwivedi and Misra [31]. Eudragit® RSPO and Eudragit® RLPO show two peaks at 1721 cm−1 and 1437 cm−1 corresponding to the carbonyl (C O) and methyl (CH3) groups respectively [32]. PEG 6000 shows a band at 1050 – 1100 cm−1 due to the stretching of the bond (C—O). The study conducted by El Maghraby and Elsergany proved the same results [33]. HPMC shows two characteristic peaks at 1900 cm−1 due to the (C—H) bond stretching and 1049 cm−1 due to the (C—O) bond stretching (strong cellulose band). Magnesium stearate shows peaks at 2916 and 2849 cm−1 as a result of the alkyl chain. Other peaks are detected at 1446 and 1570 cm−1 due to the presence of carboxylate anion. FT-IR spectrum of Avicel shows several peaks at 2913, 1426, 1368, 1314, 1161, 1049 and 896 cm−1 due to the presence of stretching bonds of CH and CH2, symmetric bending of bonds CH2, bending bond of (C—H), bending of hydroxy group (OH) in-plane, asymmetric stretching of bonds (C—O—C) (ß-glucosidic linkage), stretching of (C—O) and (C—C), and asymmetric stretching vibrations of ß-glycosidic linkage which is out of plane. Rojas et al., observed the same peaks. The FT-IR spectrum of the physical mixture reveals all peaks detected on the spectrum of the pure ETD with no shifting. There may be only decrease in the peaks intensity due to the incorporation of multiple ingredients. This could mean that there is no incompatibility between the incorporated ingredients.
Study of the surface topography
SEM photomicrographs were recorded for the surface of the optimized bilayer tablet. Results showed a clear marked interface between the fast and sustained release layers at their junction area as shown in Fig. 5C, Fig. 6. The surface of the fast release layer, represented in Fig. 5C, Fig. 6, showed large spherical particles of SSG dispersed within the surface. Fig. 5C, Fig. 6 shows HPMC fibers dispersed across the sustained release layer in the form of cylindrical shaped crystals. The sustained release layer was found to contain numerous pores that water should penetrate to allow disintegration.
In-vivo anti-inflammatory performance
The optimized formulation, C1, was assessed for its anti-inflammatory effects by performing formalin-induced swelling in rats right hind paw test. Diluted formalin solution (5% in saline) was injected in the rats’ right hind paw. It was stated that the induced inflammation produced two phases of licking the inflamed paw [34]. The tablet was placed in each rat’s mouth by a forceps, and then one milliliter of water was given to facilitate the swallowing. Due to its flexibility, oral gavage may be used in case of difficulty of swallowing. The first phase was controlled by the release of histamine and serotonin followed by kinins. The second phase was mediated by prostaglandins. Therefore, two licking phases were observed which were separated by a resting phase. The first licking phase was observed at the same time histamine was released. This occurred in the first 10 min after induction of inflammation. The second licking phase was detected at the time of prostaglandins release, this was noticed after 20 to 30 min after the induction [34].
In the current trial, three groups of rats were tested; group (A) was the “control group” that received no medication, group (B) was the “control ETD group” that received compressed ETD tablet (13.5 mg) containing PEG 6000 and Avicel PH-101 only, without any polymers affecting the drug release, and group (C) that received the optimized formulation C1. The goal of this trial was to assess the anti-inflammatory activity of ETD by comparing the maximum possible effect (%MPE) of the treatment within groups. The %MPE was calculated for each group by recording the total time spent by the rat licking or biting its inflamed right hind paw divided by the time spent by the rats in the control group (that received no medications) as mentioned before in Eq. (2). The second goal of that trial was testing the lag-time and the sustained drug release expected from the optimized formulation C1. This could be estimated by measuring the swelling degree (as in Eq. (3) of the right hind paw over a period of 6 h using the aid of Vernier caliper (APT Measuring Instrument, Omaha, USA). According to Table 4, results show that rats in group (C) spent nearly the same time of licking their inflamed right hind paws during the first phase as that of group (A). This was due to the triple coat that hindered the drug release during this phase and hence retarded the onset of drug action. The least licking time was spent by group (B), which received pure ETD. For the second licking phase, group (B) also showed the least time interval, followed by groups (A) and (C), respectively. The formulation group (C) did not show any action in inflammation elimination due to its delayed drug release. Moreover, when calculating the maximum possible effect (%MPE) for groups (B) and (C), the control ETD group showed markedly higher %MPE than the formulation group.
Table 4.
First, second, total licking time, and the maximum possible effect.
| Time (sec) | Group (A) | Group (B) | Group (C) |
|---|---|---|---|
| First licking phase | 305 ± 56.47 | 110 ± 54.68 | 257 ± 72.74 |
| Second licking phase | 109.33 ± 20.54 | 48.67 ± 9.52 | 113.67 ± 20.65 |
| Post-drug total licking | 770 ± 66.45 | 575 ± 75.65 | 750 ± 97.78 |
| %MPE | – | 25.32% | 2.60% |
Results of swelling degrees are shown in Fig. 6. They were calculated for each group of rats by the subtraction of the circumference of the rats’ right hind paw before formalin injection (zero time) from that of the same paw after formalin injection at different time points. Group (A) showed a clear increase in the swelling degree after formalin injection, in the first h and continued a gradual increase again with time. This was a result of being deprived from medication. Rats in group (B) showed an increase in the swelling degree after 30 min, but with lesser extent than the control group. Their swelling degree declined after one h reflecting the fast acting anti-inflammatory activity of ETD normal tablet. After 2 h, the swelling degree started to re-increase again. This was probably due to the beginning of drug elimination from rat’s body. The swelling degree of group (C) resembled the control group for the first h. Then, great decrease in the swelling degree occurred after two h. This was followed by more decrease across time until the end of the trial. This means that the formulation C1 remained idle for more than one h (due to the triple coat), then the fast release layer released the drug causing a sudden drop in the inflammation represented by the decrease in the swelling degree. Moreover, the sustained release layer kept releasing ETD over the whole time interval leading to an additional decrease in the swelling degree. This also explains the unnoticed %MPE of the optimized formulation C1, as it has no role in the first h corresponding to the two licking periods. One-way ANOVA test was applied to the results of the swelling degree for the three groups. The P-value and post hoc results are shown in Table 5. Results indicated that there is a significant difference (P-value < 0.05) between group (A) and each of the other two groups, while no significant difference (P-value > 0.05) between groups (B) and (C).
Table 5.
ANOVA and post hoc analysis of the in-vivo three groups.
| Groups | ANOVA P-value | Post hoc significance | |
|---|---|---|---|
| A | B | 0.011 | 0.009 |
| A | C | 0.045 | 0.042 |
| B | C | 0.482 | 0.464 |
Conclusions
The present study demonstrated the successful development, optimization and assessment of pulsatile and sustained etodolac bilayer tablets. Results showed that sodium starch glycolate, Eudragit® RSPO and HPMC K15M highly affected the rate and extent of drug release in the fast and sustained layers respectively. Successive coatings of tablets with Opadry® II, HPMC E5/K4M and Surelease® delayed the drug release with a four h lag time, pertaining to the triple coating process. Thus the formulation could bypass contact with the stomach. In-vivo response of rats clearly illustrated the chronological anti-inflammatory effect of etodolac from coated bilayer tablet formulation.
Acknowledgments
Acknowledgments
The authors are thankful to Global NAPI (GNP) Pharmaceuticals, Cairo, Egypt for providing Etodolac and Avicel PH-101, Pharco Pharmaceuticals, Alexandria, Egypt for providing croscarmellose sodium and sodium starch glycolate, Colorcon Ltd., Kent, United Kingdom for providing HPMC E5, HPMC K4M and HPMC K15M, OpadryII® and Surelease®, and Evonik Industries, North Rhine-Westphalia, Germany for providing Eudragit® RSPO and Eudragit® RLPO.
Conflict of interest
The authors report no conflict of interest.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.McInnes I.B., Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389:2328–2337. doi: 10.1016/S0140-6736(17)31472-1. [DOI] [PubMed] [Google Scholar]
- 2.Bjurström M.F., Olmstead R., Irwin M.R. Reciprocal relationship between sleep macrostructure and evening and morning cellular inflammation in rheumatoid arthritis. Psychosom Med. 2017;79:24–33. doi: 10.1097/PSY.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paolino S., Cutolo M., Pizzorni C. Glucocorticoid management in rheumatoid arthritis: Morning or night low dose? Reumatologia. 2017;55:189–197. doi: 10.5114/reum.2017.69779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellamy N. Etodolac in the management of pain: a clinical review of a multipurpose analgesic. Inflammopharmacology. 1997;5:139–152. doi: 10.1007/s10787-997-0023-8. [DOI] [PubMed] [Google Scholar]
- 5.Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology. 2001;120:594–606. doi: 10.1053/gast.2001.21907. [DOI] [PubMed] [Google Scholar]
- 6.Aronson J.K. 16th ed. Elsevier; 2006. Meyler’s side effects of drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions. [Google Scholar]
- 7.Michelucci JJ, Sherman DM, DeNeale RJ. Sustained release etodolac. US4966768A, 1990.
- 8.Momin M.M., Kane S., Abhang P. Formulation and evaluation of bilayer tablet for bimodal release of venlafaxine hydrochloride. Front Pharmacol. 2015;6:144. doi: 10.3389/fphar.2015.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maroni A., Zema L., Cerea M., Sangalli M.E. Oral pulsatile drug delivery systems. Expert Opin Drug Deliv. 2005;2:855–871. doi: 10.1517/17425247.2.5.855. [DOI] [PubMed] [Google Scholar]
- 10.Jain D., Raturi R., Jain V., Bansal P., Singh R. Recent technologies in pulsatile drug delivery systems. Biomatter. 2011;1:57–65. doi: 10.4161/biom.1.1.17717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Priyanka S., Vandana S. A review article on: superdisintegrants. Int J Drug Res Technol. 2017;3:11. [Google Scholar]
- 12.Giri T.K., Kumar K., Alexander A., Badwaik H., Tripathi D.K. A novel and alternative approach to controlled release drug delivery system based on solid dispersion technique. Bull Fac Pharmacy, Cairo Univ. 2012;50:147–159. [Google Scholar]
- 13.Chambin O., Champion D., Debray C., Rochat-Gonthier M.H., Le Meste M., Pourcelot Y. Effects of different cellulose derivatives on drug release mechanism studied at a preformulation stage. J Control Release. 2004;95:101–108. doi: 10.1016/j.jconrel.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Özkan Y., Doğanay N., Dikmen N., Işımer A. Enhanced release of solid dispersions of etodolac in polyethylene glycol. Farm. 2000;55:433–438. doi: 10.1016/s0014-827x(00)00062-8. [DOI] [PubMed] [Google Scholar]
- 15.Jallo L.J., Ghoroi C., Gurumurthy L., Patel U., Davé R.N. Improvement of flow and bulk density of pharmaceutical powders using surface modification. Int J Pharm. 2012;423:213–225. doi: 10.1016/j.ijpharm.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Barakat N.S. Etodolac-liquid-filled dispersion into hard gelatin capsules: an approach to improve dissolution and stability of etodolac formulation. Drug Dev Ind Pharm. 2006;32:865–876. doi: 10.1080/03639040500534192. [DOI] [PubMed] [Google Scholar]
- 17.Miranda H.F., Noriega V., Sierralta F., Prieto J.C. Interaction between dexibuprofen and dexketoprofen in the orofacial formalin test in mice. Pharmacol Biochem Behav. 2011;97:423–427. doi: 10.1016/j.pbb.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Osman H.F., Atya A.M. Influence of electrolytes supplementation on cardiac and renal functions after prolonged exercise in male rats. World Appl Sci J. 2013;23:1377–1385. [Google Scholar]
- 19.Lu W., Luo H., Zhu Z., Wu Y., Luo J., Wang H. Preparation and the biopharmaceutical evaluation for the metered dose transdermal spray of dexketoprofen. J Drug Deliv. 2014;2014 doi: 10.1155/2014/697434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadam P.S., Pande V.V., Vibhute S.K., Giri M.A. Exploration of mixed hydrotropy strategy in formulation and development of etodolac injection. J Nanomedicine Res. 2016;3:63. [Google Scholar]
- 21.Vraníková B., Gajdziok J., Doležel P. The effect of superdisintegrants on the properties and dissolution profiles of liquisolid tablets containing rosuvastatin. Pharm Dev Technol. 2017;22:138–147. doi: 10.3109/10837450.2015.1089900. [DOI] [PubMed] [Google Scholar]
- 22.Kuksal A., Tiwary A.K., Jain N.K., Jain S. Formulation and in vitro, in vivo evaluation of extended-release matrix tablet of zidovudine: influence of combination of hydrophilic and hydrophobic matrix formers. AAPS Pharmscitech. 2006;7:E1–E9. doi: 10.1208/pt070101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandav S., Naik J. Sustained release of ramipril from ammonio methacrylate copolymer matrix prepared by high pressure homogenizer. Int J Pharm Pharm Sci. 2014;6:349–353. [Google Scholar]
- 24.Shagufta K., Kishor C., Dilesh S., Pramod Y. Formulation and release behavior of sustained release Stavudine Hydrochloride Matrix tablet containing hydrophilic and hydrophobic polymers. Int J Drug Dev Res. 2013;5:32–37. [Google Scholar]
- 25.Patel A.R., Schatteman D., Lesaffer A., Dewettinck K. A foam-templated approach for fabricating organogels using a water-soluble polymer. Rsc Adv. 2013;3:22900–22903. [Google Scholar]
- 26.Kazlauske J., Cafaro M.M., Caccavo D., Marucci M., Lamberti G., Barba A.A., et al. Determination of the release mechanism of Theophylline from pellets coated with Surelease®—A water dispersion of ethyl cellulose. Int J Pharm. 2017;528:345–353. doi: 10.1016/j.ijpharm.2017.05.073. [DOI] [PubMed] [Google Scholar]
- 27.Ali R., Dashevsky A., Bodmeier R. Poly vinyl acetate and ammonio methacrylate copolymer as unconventional polymer blends increase the mechanical robustness of HPMC matrix tablets. Int J Pharm. 2017;516:3–8. doi: 10.1016/j.ijpharm.2016.10.069. [DOI] [PubMed] [Google Scholar]
- 28.Liu Q., Gong Y., Shi Y., Jiang L., Zheng C., Ge L., et al. A novel multi-unit tablet for treating circadian rhythm diseases. AAPS PharmSciTech. 2013;14:861–869. doi: 10.1208/s12249-013-9975-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiss D., Zelkó R., Novák C., Éhen Z. Application of DSC and NIRS to study the compatibility of metronidazole with different pharmaceutical excipients. J Therm Anal Calorim. 2006;84:447–451. [Google Scholar]
- 30.Mishra B., Arya N., Tiwari S. Investigation of formulation variables affecting the properties of lamotrigine nanosuspension using fractional factorial design. Daru. 2010;18:1–8. [PMC free article] [PubMed] [Google Scholar]
- 31.Dwivedi A., Misra N. Quantum chemical study of Etodolac (Lodine) Der Pharma Chem. 2010;2:58–65. [Google Scholar]
- 32.Ben E.S., Nofita R., Rusdi S., Suardi M., Djamaan A. Use of eudragit RS PO in the formulation of acyclovir hollow-microspheres by solvent evaporation technique. Der Pharm Lett. 2016;8:53–59. [Google Scholar]
- 33.El Maghraby G.M., Elsergany R.N. Fast disintegrating tablets of nisoldipine for intra-oral administration. Pharm Dev Technol. 2014;19:641–650. doi: 10.3109/10837450.2013.813543. [DOI] [PubMed] [Google Scholar]
- 34.Hunskaar S., Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]