Graphical abstract
Keywords: Wheat, Aluminum, Auxin, Nitrate, Arginine, Antioxidant enzymes
Highlights
-
•
Endogenous NO levels are higher in roots of Al-tolerant wheat.
-
•
NO precursors decrease Al accumulation in wheat root cells.
-
•
NO precursors stimulate auxin flow towards roots in Al-treated wheat.
-
•
Al-induced oxidative stress is attenuated in wheat roots by NO precursors.
-
•
Increased endogenous NO content contribute, in part, to wheat Al tolerance.
Abstract
Aluminum (Al) is an element widely distributed in soils, even though Al3+ is one of the most detrimental cations to plant growth. The effect of nitric oxide (NO) precursors on indole-3-acetic acid (IAA) flow towards roots upon Al treatment is herein reported using two Triticum aestivum (wheat) cultivars with recognized differential Al tolerance. Roots of Al-tolerant seedlings with no treatment (control) accumulated higher amounts of NO than Al-sensitive ones. The treatment with Al further stimulated NO production in root cells while root exposure to NO3−, L-arginine (Arg) or the NO donor S-nitrosoglutathione (GSNO) decreased both Al and lipid peroxide accumulation in both cultivars. Regardless of the cultivar, NO3−, Arg or GSNO prevented the blockage of IAA flow towards roots. Overall, the treatment of wheat roots with NO precursors prior to Al treatment effectively guarantees normal IAA flow towards roots, a condition that favors the organ’s growth and development.
Introduction
Aluminum (Al) is a metallic element widely distributed in soils. The pH affects Al availability and acid soils (pH ≤ 5) have Al in the soluble form (Al3+), which can be promptly taken up by plant root systems. Indeed, the presence of bioavailable Al is one of the major limitations for crop growth in acid soils [1], [2].
It is well known that even at relatively low concentrations, Al3+ may affect plants growth and development [2]. The distal part of the transition zone in roots was found to be the primary Al target, which results in impaired root growth [1], [3] and plant development [4]. Common symptoms of Al damage to roots include the appearance of a yellowish or dark coloration at the tips due to oxidation of phenolic compounds and the formation of lateral-branched, tortuous and thickened roots devoid of the development of absorbent root hairs [5]. Anatomically, a phenomenon that also contributes to this is the wrinkling or even the collapse of root cells as a function of the Al concentration and exposure time. Indeed, a decrease in the number of cells undergoing division and their arrangement in Al-treated root tips has been recorded [5], [6]. As a result, both absorption and transport of water and nutrients were seriously affected, compromising the plant development [7].
The disruption of polar auxin transport from shoots to root apices has been demonstrated to be one of the effects of Al that drives the inhibition of root elongation [8]. The transport of PIN2 vesicles between the plasma membrane and endosome was disrupted by Al in Arabidopsis roots that, in turn, caused a decrease by 34% of indole-3-acetic acid (IAA; an auxin) transport to the root apex [9]. Accordingly, the treatment of the elongation zone of maize roots with the auxin indole-3-acetic acid (IAA) reestablished root growth in the presence of Al [8].
Plants have several strategies to respond to Al stress, which includes Al exclusion from roots and enhancement of their antioxidant system [10], [11]. Although the mechanisms of Al toxicity and tolerance are still not completely understood, it is known that Al inhibits the signaling cascade related to inositol 1,4,5-trisphosphate in Triticum aestivum (wheat) roots [12]. Several signaling molecules, including nitric oxide (NO), were identified to be involved in Al tolerance by plants [13], [14]. NO is a free radical that has emerged as a signaling molecule in plant cells during seed germination, root growth and development, photomorphogenesis, cell death, senescence and response to abiotic and biotic stresses [15], [16], [17], [18], [19], [20], [21], [22]. The NO produced from nitrate reductase (NR) activity was suggested to account for the tolerance of Phaseolus vulgaris (red kidney bean) to Al as this free radical decreased reactive oxygen species (ROS) and lipid hydroperoxide levels [23]. The NR mediated an early NO burst, which plays a key role in Al resistance of wheat through modulating antioxidant defense [24]. Putrescine-induced NR activity and NO production was reported to enhance Al tolerance in red kidney bean via modulation of citrate secretion from roots [25]. Additionally, the endosomal compartmentalization of Al was accompanied by a decreased NO biosynthesis in the root apex [3].
The role of exogenous NO in the response of root cells to Al has been disclosed. The use of sodium nitroprusside (SNP), an NO donor, decreased Al accumulation in Cassia tora roots after Al treatment [26] and restored root elongation in Al-treated Hibiscus moscheutos (rose mallow) [13]. Modulation of the activity of apoplastic peroxidases was observed in Al-challenged Cassia tora upon SNP treatment [27]. Exogenous NO was implicated in the regulation of hormone equilibrium to different extents in root apices in response to Al in wheat cv. Jinmai47 (Al-sensitive) and Secale cereale cv. King (rye; Al-tolerant) [28]. As observed for SNP, the NO donor S-nitrosoglutathione (GSNO) attenuated the Al-triggered growth inhibition of roots of wheat cvs. Yang-5 (Al-sensitive) and Jian-864 (Al-tolerant) [29]. Indeed, IAA content increased in wheat root apices, but not in rye root apices in response to Al after SNP treatment [28]. Furthermore, a body of evidence suggests that NO can be directly or indirectly originated in plant cells from NO3−, NO2− and/or L-arginine (Arg) [17], [19], [20], [30], [31], which make them precursors of NO in plant cells.
To expand the knowledge on the extent of NO production and its effect on IAA transport to plant roots in response to Al, it was investigated the ability of the NO precursors NO3− and Arg and the NO donor GSNO to prevent Al-caused IAA flow blockage towards wheat roots. Two wheat cultivars with differential tolerance to Al were used to check the hypothesis and verify whether NO precursors successfully boost the enzymatic antioxidant system and mitigate lipid and protein oxidations in roots upon Al stress. Additionally, experiments of in situ histochemical localization of NO in root apices were performed to verify if the pattern of NO distribution in root cells is associated with the innate Al tolerance in wheat.
Material and methods
Plant material and treatments
Experiments were performed with seedlings of wheat cvs. Anahuac (Al-sensitive) and BH1146 (Al-tolerant) [32], whose seeds were kindly supplied by Dr. Antônio Wilson Penteado Ferreira Filho from the Agronomic Institute of Campinas (IAC), SP, Brazil.
Seeds were surface sterilized with 1% NaClO for 5 min, washed with deionized water and lined up on Whatman™ paper (15 seeds per paper) imbibed with 0.2% Nystatin® aqueous solution to prevent fungal contamination. Whatman™ paper was folded to obtain a cylinder that was transferred to a water-containing beaker and kept in a Bio-Oxygen Demand chamber set to 25 °C and 12 h photoperiod. After four days, seedlings were rinsed with deionized water, transferred to 500 mL-pots (6 seedlings per pot) and hydroponically maintained for 24 h under the following treatments: H2O (control); 300 µM NO3−; 300 µM Arg or 300 µM GSNO. Afterwards, roots were washed, incubated with 200 µM Ca2+ (provided as CaCl2; pH 4.0) or 75 µM AlCl3 (pH 4.0) in 200 µM Ca2+ for 48 h and harvested for the analyses. The concentration of Al3+ in the solution was not determined. It is known that Al species other than Al3+ form at pH below 5.0 [32], [33], such as AlOH2+ and Al(OH)2+.
Detection of Al and NO in root tissues
For Al histochemical localization, sections of fresh root apices were incubated with 100 μM morin in acetate buffer (pH 5.0) for 1 h, washed and observed with an epi-fluorescence microscope (Olympus BX41 equipped with Olympus FITC filters and an Olympus SC30 digital camera) using excitation and emission wavelengths of 450 and 570 nm, respectively [34]. For NO localization, sections of fresh root apices were incubated with 4,5-diaminofluorescein diacetate (DAF-2DA; 10 µM) [18] for 15 min, washed, mounted in Vectashield® and observed at the same epi-fluorescence microscope (excitation at 450 nm; emission at 570 nm). The amounts of Al and NO accumulated in root apices were estimated in terms of percentage of pixels by measuring the green fluorescence intensity in the freehand sections using the software ImageJ (imagej.nih.gov/ij/download.html).
Immunohistochemical localization of IAA
An immunohistochemical approach was used to localize IAA in tissues of fresh-collected root tip 5-mm-segments [35], [36], [37]. The segments were pre-fixed at 4 °C for 4 h in 4% 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDAC), followed by post-fixation in 10 mM phosphate buffer (pH 7.2) containing 5% glutaraldehyde and 4% formaldehyde [38] at 4 °C for 16 h. The fixed tissues were then dehydrated in a butanol series, embedded in Paraplast® at 60 °C and sectioned at 10 μm. After the paraffin was removed, the sections were rehydrated and incubated for 45 min in a blocking solution constituted of 10 mM phosphate-buffered saline (PBS; pH 7.0), 0.1% tween-20, 1.5% glycine and 5% BSA. After washing with a 10 mM PBS/0.1% tween-20/0.8% BSA solution, the sections were incubated overnight at 4 °C with 50 µg mL−1 monoclonal anti-auxin antibody produced in mouse (Sigma). Sections were washed with a mixture of 10 mM PBS and 0.8% BSA and further incubated with the secondary antibody anti-mouse IgG alkaline phosphatase conjugate (20-fold diluted; Sigma) for 7 h at room temperature. After washing with the diluent solution, sections were developed in 0.34 g L−1 nitro blue tetrazolium (NBT)/0.18 g L−1 5-bromo-4-chloro-3-indolyl phosphate (BCIP) until formation of a bluish color. A stop buffer [100 mM Tris (pH 8.0) plus 1 mM EDTA] was used to rinse the sections that were further mounted in Mounting Medium and observed at a Leica DM500 Optical Microscope equipped with a Leica ICC50 HD camera. In addition to the usual treatments, roots were treated for 24 h with 2,3,5-triiodobenzoic acid (TIBA; 10 μM), an inhibitor of auxin transport, alone or in combination with 300 µM GSNO prior to the exposure (or not) to Al for 48 h.
Quantification of lipid hydroperoxides (LOOH) and oxidized proteins
Roots were ground in liquid nitrogen in the presence of polyvinylpolypyrrolidone (PVPP). Total lipids were extracted with 80% ethanol containing butylated hydroxytoluene (BHT; 1 mL/0.3 g plant material) and quantified using a ferrous oxidation-xylenol orange (FOX) reagent essentially as described elsewhere [39]. The LOOH contents were determined as hydrogen peroxide equivalents g−1 dry weight using a standard curve prepared with the commercial substance.
As for the quantification of oxidized proteins, roots were ground in liquid nitrogen in the presence of PVPP for the extraction of soluble proteins with 50 mM HEPES (pH 7.0). Homogenates were centrifuged at 10,000g for 10 min at 4 °C. A 1.5-vol of supernatant was added to 1-vol of 8 mM 2,4-dinitrophenylhydrazine/2 M HCl and after incubation for 1 h in the dark, a half-volume of 30% trichloroacetic acid (w/v) was added to each reaction. The solution was centrifuged at 10.000g for 10 min, the supernatants were disposed of, the pellets washed 3 times with ethanol/ethyl acetate (1:1) and resuspended in 6 M guanidine/20 mM phosphate buffer (pH 2.3). Samples were analyzed at 360 nm and the levels of oxidized proteins expressed as nmols of carbonyl equivalents mg−1 total protein.
Antioxidant enzymes assay
Root samples were ground in liquid nitrogen in the presence of PVPP. Soluble proteins were extracted with 50 mM phosphate buffer (pH 6.8) containing 100 µM EDTA and protease inhibitor cocktail (Sigma). Superoxide dismutase (SOD) activity was measured by incubating plant homogenates with 50 mM phosphate buffer (pH 7.8), 13 mM L-methionine, 100 µM EDTA, 2 µM riboflavin and 75 µM NBT [40]. Reactions were maintained for 10 min at room temperature in a chamber equipped with a 15 W fluorescent light. Control reactions were carried out under darkness. The blue formazan derived from NBT reduction was quantified at 575 nm. One unit of SOD activity was defined as the amount of enzyme required to inhibit NBT reduction by 50%. Catalase (CAT) activity was determined from incubations of plant homogenates with 50 mM phosphate buffer (pH 6.8) in the presence of 125 µM H2O2 [41]. The H2O2 degradation was monitored at 240 nm and CAT activity calculated using the molar extinction coefficient of 39.4 M−1 cm−1. Ascorbate peroxidase (APX) activity was determined by incubating root homogenates with 50 mM phosphate buffer (pH 6.0), 1 mM ascorbic acid and 2 mM H2O2 [42]. The oxidation of ascorbic acid was monitored at 290 nm and APX activity expressed as µmol of consumed ascorbate min−1 mg−1 protein.
Protein contents were determined by the Coomassie Blue-binding method [43], using BSA as the standard.
Statistical analysis
Data were analyzed by one-way ANOVA. Comparison of means of different treatments in the same cultivar was performed by Tukey test at 5% significance level while the effect of each treatment on the cultivars was assessed by Student’s t-test at a 5% significance level.
Results
Histochemical localization of Al in root
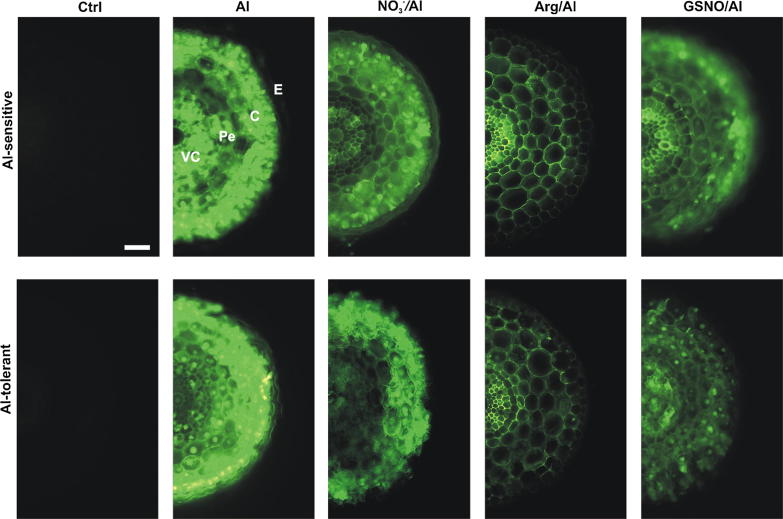
As expected, after incubation with morin, control roots (Ctrl) of wheat seedlings from both cultivars did not exhibit any green fluorescence typical of Al complexation to this flavonoid (Fig. 1). The pattern of Al accumulation in both wheat cultivars was distinct from one another; Al-sensitive seedlings presented Al all over root tissues from epidermis to vascular cylinder while the metal cation was mainly detected in epidermal and cortex cells of Al-tolerant roots (Fig. 1, Fig. 2). The intensity of green fluorescence in the Al-exposed root of both cultivars in seedlings decreased upon pre-treatment with Arg or NO3−; application of Arg or NO3− yielded 85.3 and 73.9% less Al in Al-sensitive roots and 10.9 and 40.2% less Al in Al-tolerant ones (Fig. 1; Table 1). In addition, Al was detected mainly in cortex cells of roots pre-treated with NO3− or Arg and in epidermal cells in NO3−-exposed roots. The treatment with NO3− prevented Al accumulation in the vascular cylinder of the Al-sensitive cultivar. The Arg treatment lowered Al accumulation in the root epidermis of both cultivars but did not prevent Al accumulation in the vascular cylinder of Al-tolerant roots (Fig. 1). The Al distribution in roots also changed upon pre-treatment with the NO donor GSNO. In Al-sensitive roots green fluorescence was exhibited in the epidermal and vascular cylinder cells while only a few of the epidermal, cortex, and vascular cylinder cells of Al-tolerant roots displayed green fluorescence (Fig. 1). The Al-derived green fluorescence was decreased by 9% in Al-tolerant roots treated with GSNO while an increment of 15% was recorded in roots of Al-sensitive seedlings under similar treatment (Table 1).
Fig. 1.
Localization of Al in wheat root apices using morin. Four-day-old seedlings of cultivars Anahuac (Al-sensitive) and BH1146 (Al-tolerant) were hydroponically incubated with 200 µM Ca2+ (Ctrl) or 75 µM AlCl3 in 200 µM Ca2+ for 48 h. Alternatively, seedlings were pre-treated with NO3−, L-arginine (Arg) or S-nitrosoglutathione (GSNO) at 300 µM for 24 h followed by exposure to Al for 48 h. Green fluorescence indicates presence of Al. VC, vascular cylinder; Pe, pericycle; C, cortex; E, epidermis. Images are representative of at least two experiments, each done in triplicate. Bar = 100 µm.
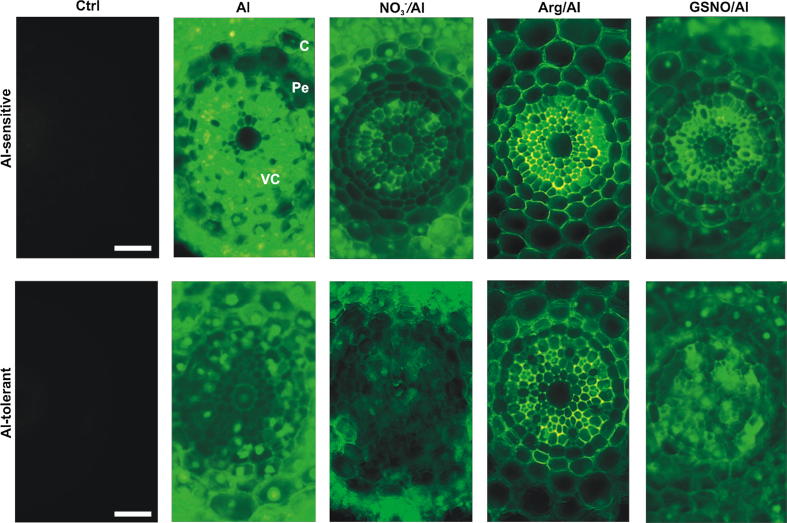
Fig. 2.
Localization of Al in the stele of wheat root apices using morin. Images correspond to magnifications of those shown in Fig. 1 to better visualize Al in vascular cylinder and neighborhood. VC, vascular cylinder; Pe, pericycle; C, cortex. Images are representative of at least two experiments, each done in triplicate. Bars = 25 µm.
Table 1.
Aluminum (Al) and nitric oxide (NO) content in wheat root apices. Four-day-old seedlings of cultivars Anahuac (Al-sensitive) and BH1146 (Al-tolerant) were hydroponically incubated with 200 µM Ca2+ (Ctrl) or 75 µM AlCl3 in 200 µM Ca2+ for 48 h. Alternatively, seedlings were pre-treated with NO3−, L-arginine (Arg) or S-nitrosoglutathione (GSNO) at 300 µM for 24 h followed by exposure to Al for 48 h. Values correspond to the percentage of green fluorescence pixels present in the images of Fig. 1, Fig. 3.
| Treatments | Al (%) |
NO (%) |
||
|---|---|---|---|---|
| Anahuac | BH1146 | Anahuac | BH1146 | |
| Ctrl | 0.0 | 0.0 | 15.5 | 42.6 |
| Al | 43.0 | 38.0 | 29.9 | 34.8 |
| NO3−/Al | 38.3 | 22.7 | 35.0 | 38.4 |
| Arg/Al | 6.3 | 9.9 | 36.8 | 30.4 |
| GSNO/Al | 49.7 | 34.6 | 48.4 | 32.2 |
Histochemical localization of NO in root
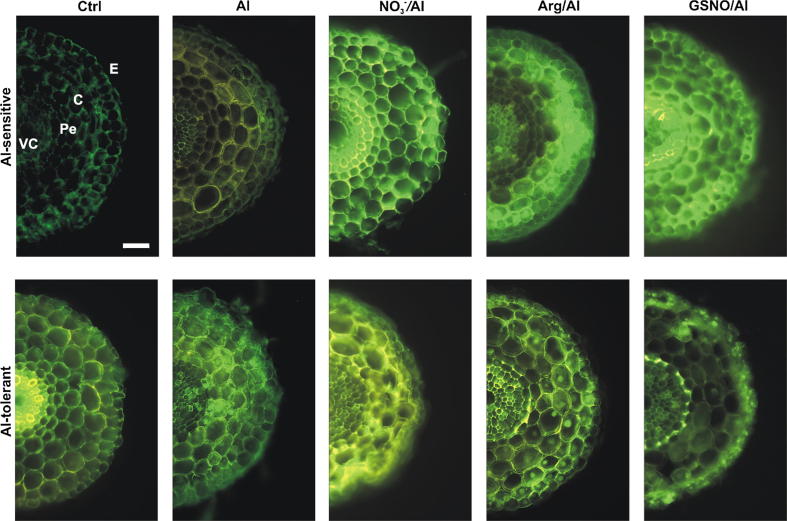
The presence of NO in root tissues was confirmed by the formation of triazolo-fluorescein (a green fluorescent molecule), after incubation of cross-sections with DAF-2DA. Some green fluorescence was detected in the epidermis and cortex of Al-sensitive control roots while a very intense green fluorescence was observed in the vascular cylinder of Al-tolerant control roots (Fig. 3, Fig. 4). The NO content in control Al-tolerant roots apices was at least 2.7-fold higher than that of Al-sensitive roots apices under comparable conditions (Table 1). Upon Al treatment, NO formation increased 1.9-fold in Al-sensitive roots, with distribution in epidermal and cortex cells. The NO production decreased by 18% in Al-tolerant roots exposed to Al in which, the very intense fluorescence originally observed in the vascular cylinder of control roots is now distributed throughout cells of outer tissues (Fig. 3; Table 1). Pre-treatment with NO3− or Arg caused NO accumulation in almost all tissues of Al-exposed roots in comparison to those seedlings solely treated with this metal cation, regardless of the cultivar (Fig. 3). Whereas NO levels increased by 17, 23 and 62% in Al-treated Al-sensitive roots pre-treated with NO3−, Arg or GSNO, respectively, a 10% increment was observed in Al-tolerant roots pre-treated with NO3− and a 12 and 7.4% decrease of NO content was shown in these roots pre-treated with Arg or GSNO, respectively (Table 1). Interestingly, the treatment of roots with GSNO yielded NO in all tissues of Al-sensitive roots while an intense green fluorescence typical of NO presence was detected only in pericycle and epidermal cells of Al-tolerant roots (Fig. 3).
Fig. 3.
Localization of NO in wheat root apices using DAF-2DA. Four-day-old seedlings of cultivars Anahuac (Al-sensitive) and BH1146 (Al-tolerant) were hydroponically incubated with 200 µM Ca2+ (Ctrl) or 75 µM AlCl3 in 200 µM Ca2+ for 48 h. Alternatively, seedlings were pre-treated with NO3−, L-arginine (Arg) or S-nitrosoglutathione (GSNO) at 300 µM for 24 h followed by exposure to Al for 48 h. Green fluorescence indicates presence of NO. VC, vascular cylinder; Pe, pericycle; C, cortex; E, epidermis. Images are representative of at least two experiments, each done in triplicate. Bar = 100 µm.
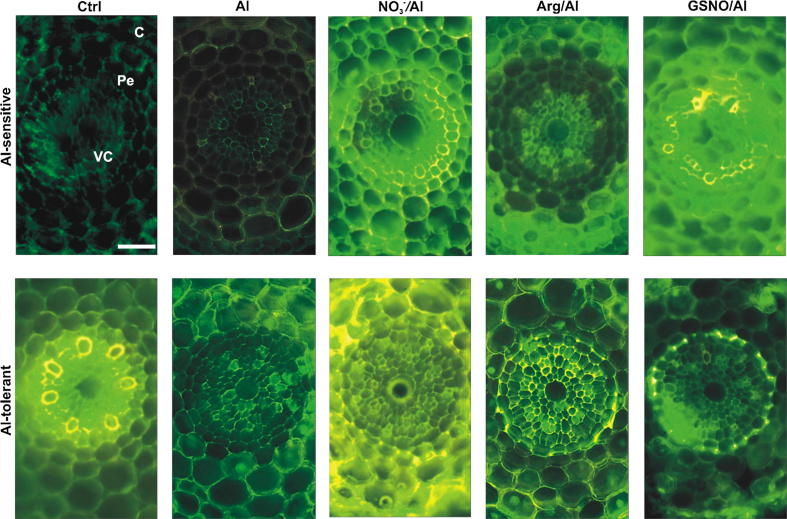
Fig. 4.
Localization of NO in the stele of wheat root apices using DAF-2DA. Images correspond to magnifications of those shown in Fig. 3 to better visualize NO in vascular cylinder and neighborhood. VC, vascular cylinder; Pe, pericycle; C, cortex. Images are representative of at least two experiments, each done in triplicate. Bars = 25 µm.
Immunohistochemical localization of IAA
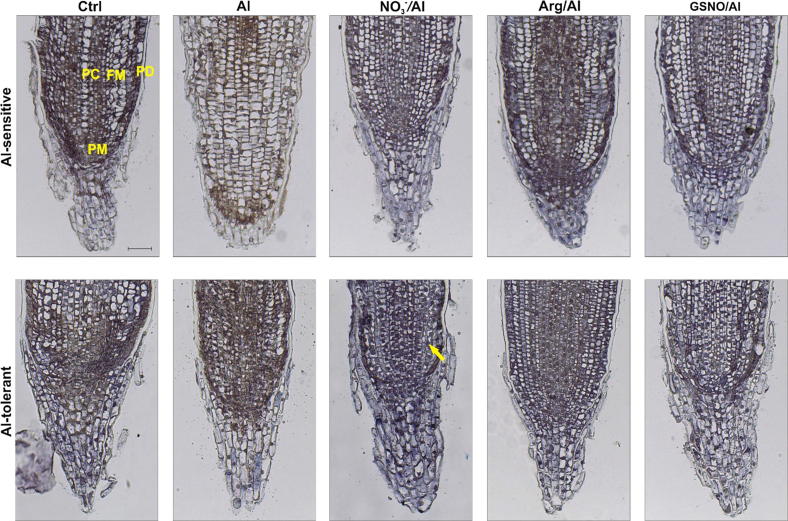
The presence of IAA in root cells was characterized by the formation of blue spots. The IAA was detected in the protoderm, procambium and promeristem cells of control (Ctrl) roots, regardless of the plant cultivar (Fig. 5). However, the intensity of the blue color was especially high in the Al-tolerant cultivar in the procambium and promeristem of Ctrl roots. The Al negatively affected the distribution of IAA in roots of Al-sensitive and Al-tolerant cultivars by 30 and 20%, respectively (Fig. 5; Table 2).
Fig. 5.
Immunolocalization of auxin in wheat root apices. Four-day-old seedlings of cultivars Anahuac (Al-sensitive) and BH1146 (Al-tolerant) were hydroponically incubated with 200 µM Ca2+ (Ctrl) or 75 µM AlCl3 in 200 µM Ca2+ for 48 h. Alternatively, seedlings were pre-treated with NO3−, L-arginine (Arg) or S-nitrosoglutathione (GSNO) at 300 µM for 24 h followed by exposure to Al for 48 h. Blue spots, indicated by the arrow, represent the presence of indole-3-acetic acid (IAA). PM, promeristem; PC, procambium; FM, fundamental meristem; PD, protoderm. Images are representative of experiments done in sextuplicate. Bar = 200 µm.
Table 2.
Indole-3-acetic acid (IAA) content in wheat root apices. Four-day-old seedlings of cultivars Anahuac (Al-sensitive) and BH1146 (Al-tolerant) were hydroponically incubated with 200 µM Ca2+ (Ctrl) or 75 µM AlCl3 in 200 µM Ca2+ for 48 h. Alternatively, seedlings were pre-treated for 24 h with NO3−, L-arginine (Arg) or S-nitrosoglutathione (GSNO) at 300 µM or 2,3,5-triiodobenzoic acid (TIBA; 10 μM) alone or in combination with 300 µM GSNO followed by exposure to Al or Ca2+ for 48 h. Values correspond to the percentage of blue color pixels present in the images of Fig. 5, Fig. 6.
| Treatments | IAA (%) |
|
|---|---|---|
| Anahuac | BH1146 | |
| Ctrl | 13.0 | 16.9 |
| Al | 9.0 | 13.4 |
| NO3−/Al | 17.6 | 19.4 |
| Arg/Al | 26.6 | 22.2 |
| GSNO/Al | 16.0 | 29.5 |
| TIBA + CaCl2 | 3.5 | 11.2 |
| GSNO + TIBA + CaCl2 | 7.4 | 12.6 |
| TIBA + Al | 6.0 | 12.5 |
| GSNO + TIBA + Al | 14.5 | 17.5 |
Both NO3− and Arg pre-treatments restored or even induced IAA flow in the roots of both cultivars after Al treatment (Table 2). Under these treatments, the formation of blue spots was intense in promeristem and procambial cells, although some staining was also detected in the fundamental meristem cells (Fig. 5). The GSNO also induced the IAA flow in roots of the Al-sensitive and Al-tolerant cultivars by 23 and 75%, respectively (in relation to the control), in which IAA was detected mainly in epidermal and procambial cells (Fig. 5).
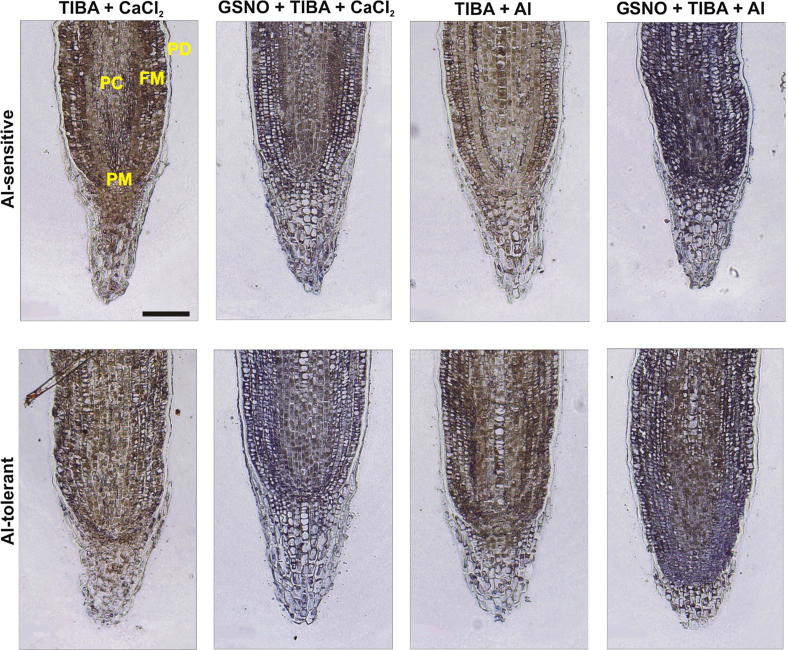
Roots from both cultivars pre-treated with TIBA, an inhibitor of auxin transport, presented IAA levels (Fig. 6; treatments TIBA + CaCl2) that were 73% (for Al-sensitive) and 33.7% (for Al-tolerant) lower than those observed in the respective control plants devoid of TIBA treatment (Fig. 6; Table 2). Exposure to Al (treatments TIBA + Al) further decreased the number of blue spots in both cultivars. Notably, the effects of TIBA on IAA transport were suppressed by the pre-treatment of roots with GSNO (treatments GSNO + TIBA + CaCl2 or GSNO + TIBA + Al). The IAA was localized in the protoderm, promeristem and procambium of roots under these treatments (Fig. 6). As expected, TIBA impaired the development of the root elongation zone as visualized by a decrease in the diameter of this region in comparison to control roots. The IAA was localized in protoderm, promeristem and procambium of roots under these treatments (Fig. 6).
Fig. 6.
Effect of an NO donor on auxin distribution in wheat root apices treated with an inhibitor of auxin transport. Four-day-old seedlings of cultivars Anahuac (Al-sensitive) and BH1146 (Al-tolerant) were hydroponically incubated with 200 µM Ca2+ (Ctrl) or 75 µM AlCl3 in 200 µM Ca2+ for 48 h. Alternatively, seedlings were pre-treated with 2,3,5-triiodobenzoic acid (TIBA) at 10 μM or S-nitrosoglutathione (GSNO) at 300 µM or 10 μM TIBA plus 300 µM GSNO for 24 h followed by exposure (or not) to Al for 48 h. Blue spots indicate presence of indole-3-acetic acid (IAA). PM, promeristem; PC, procambium; FM, fundamental meristem; PD, protoderm. Images are representative of experiments done in sextuplicate. Bar = 200 µm.
Quantification of lipid hydroperoxides (LOOH) and oxidized proteins
Control roots of both cultivars presented comparable LOOH levels (450 nmol g−1 DW) that were higher than those of Al pre-treated roots (Table 3). Upon Al treatment, LOOH levels in the Al-sensitive and Al-tolerant roots decreased 12.1% and 35.6%, respectively (Table 3). The NO3− pre-treatment further decreased LOOH content in Al-sensitive roots without affecting that of Al-tolerant ones. Both Arg and GSNO pre-treatments decreased LOOH contents in Al-treated roots of both cultivars (Table 3). The lowest levels of LOOH were observed in Al-sensitive roots treated with NO3− or Arg, while the lowest LOOH values were found in Al-tolerant roots treated with Arg or GSNO. Oxidized proteins levels in Al-tolerant Ctrl roots was twice as much as that of control Al-sensitive roots. Moreover, neither Al incubation nor any pre-treatment affected the oxidized protein levels in their roots (Table 3).
Table 3.
Effect of NO precursors on the levels of lipid hydroperoxide and oxidized proteins in wheat root challenged with Al.
| Treatments | Al-sensitive cultivar |
Al-tolerant cultivar |
||
|---|---|---|---|---|
| Number of samples | LOOH (nmol g DW−1) | Number of samples | LOOH (nmol g DW−1) | |
| Ctrl | 6 | 443.9 ± 34.8 aA | 6 | 451.5 ± 36.5 aA |
| Al | 6 | 390.4 ± 10.0 bB | 6 | 290.6 ± 5.7 bC |
| NO3−/Al | 6 | 161.7 ± 5.3 dD | 6 | 280.2 ± 18.6 bC |
| Arg/Al | 6 | 202.0 ± 26.9 dD | 6 | 199.6 ± 11.2 cD |
| GSNO/Al | 6 | 256.6 ± 19.7 cC | 6 | 173.2 ± 20.4 cD |
| Number of samples | Protein carbonyl (nmol mg protein−1) | Number of samples | Protein carbonyl (nmol mg protein−1) | |
| Ctrl | 5 | 14.4 ± 2.5 eG | 5 | 32.1 ± 1.0 eF |
| Al | 5 | 17.2 ± 1.6 eG | 8 | 29.7 ± 1.2 eF |
| NO3−/Al | 5 | 18.2 ± 0.8 eG | 5 | 32.7 ± 3.5 eF |
| Arg/Al | 5 | 15.6 ± 1.6 eG | 5 | 30.2 ± 2.0 eF |
| GSNO/Al | 5 | 21.9 ± 0.5 eG | 5 | 34.3 ± 1.8 eF |
Values are the means + SE. Arg, L-arginine; GSNO, S-nitrosoglutathione; DW, dry weight; LOOH, lipid hydroperoxide. Distinct lowercase letters indicate significant difference among treatments within a cultivar while distinct uppercase letters indicate significant difference for a certain treatment between cultivars.
Antioxidant enzymes assay
The activity of SOD in Al-tolerant roots under physiological conditions (Ctrl) was 10% higher than that of Al-sensitive roots (Table 4). The Al stimulated SOD activity by 18.4% in roots of Al-tolerant seedlings while the activity of this enzyme remained unaffected in Al-sensitive roots under the same experimental condition. The Al stimulated SOD activity by 49.1% in NO3− pre-treated Al-sensitive roots, while the enzyme activity decreased by 25.3% in Al-tolerant roots upon the same conditions. The SOD activity in Al-sensitive roots increased 19% upon pre-treatment with Arg. An increment of 85.5% in SOD activity was recorded in Al-tolerant roots with Arg pre-treatment when compared to that of roots treated solely with Al. The Al did not affect SOD activity in Al-sensitive roots pre-treated with GSNO but induced the activity of the enzyme in Al-tolerant roots under the same conditions (Table 2). The CAT activity in control Al-tolerant roots was 18% higher than that of Al-sensitive roots (Table 4). The activity of CAT further increased upon exposure of Al-tolerant roots to Al while this cation exerted no effect on the CAT activity in Al-sensitive roots. The CAT activity in Al-sensitive roots was stimulated by Al in NO3− (28%) and Arg (8.5%) treated seedlings. On the other hand, CAT activity decreased by 39.8% (in average) in NO3− and GSNO pre-treated Al-tolerant roots exposed to Al. The abiotic stress led to a 60% increment in CAT activity in Al-tolerant roots pre-treated with Arg (Table 4). As noticed for SOD and CAT, the basal APX activity in Al-tolerant roots was higher than that of Al-sensitive roots (Table 2). Overall, the effect of the treatments on APX activity in both cultivars was like that observed for CAT activity, except for the Arg treatment; the Arg decreased APX activity in Al-sensitive roots without affecting the activity of this enzyme in Al-tolerant ones (Table 4).
Table 4.
Effect of NO precursors on the activity of superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) in wheat root challenged with Al.
| Treatments | Al-sensitive cultivar |
||
|---|---|---|---|
| SOD |
CAT |
APX |
|
| (U min−1 mg prot−1) | (µmol H2O2 min−1 mg prot−1) | (µmol ASC min−1 mg prot−1) | |
| Ctrl | 27.5 ± 0.7 aA | 402.9 ± 17.5 aA | 59.7 ± 2.1 aA |
| Al | 27.4 ± 1.1 aA | 417.4 ± 18.1 aA | 60.8 ± 2.3 aA |
| NO3−/Al | 41.0 ± 1.5 bC | 534.5 ± 12.1 bC | 78.5 ± 1.7 bC |
| Arg/Al | 33.8 ± 0.7 cC | 453.0 ± 30.2 aAB | 35.4 ± 2.0 dE |
| GSNO/Al | 26.7 ± 0.9 aA | 336.2 ± 9.1 cD | 49.7 ± 1.0 cD |
| Al-tolerant cultivar | |||
| Ctrl | 30.4 ± 0.7 aB | 475.0 ± 15.7 aB | 68.5 ± 1.8 aB |
| Al | 36.0 ± 1.4 bC | 555.1 ± 2.6 bC | 80.5 ± 1.4 bC |
| NO3−/Al | 26.9 ± 1.0 cA | 337.1 ± 11.7 cD | 51.2 ± 1.7 cD |
| Arg/Al | 64.8 ± 4.6 dD | 889.5 ± 29.5 dE | 69.4 ± 2.8 aB |
| GSNO/Al | 26.2 ± 1.6 cA | 330.7 ± 18.4 cD | 50.7 ± 2.6 cD |
Values are the means + SE (n = 4). Arg, L-arginine; GSNO, S-nitrosoglutathione; ASC, ascorbate. Distinct lowercase letters indicate significant difference among treatments within a cultivar while distinct uppercase letters indicate significant difference for a same treatment between cultivars.
Discussion
Notably, the incubation of roots of both wheat cultivars with NO precursors (NO3− or Arg) led to lower levels of Al in cells than those of Al-treated roots suggesting that both molecules were metabolized to form NO that in turn, decreased Al uptake by roots (Fig. 1; Table 1). Indeed, exogenous NO was shown to decrease pectin levels and increase the activity of xyloglucan endotransglucosylase (important for cell expansion) in Arachis hypogaea, thus inhibiting Al adsorption to the cell wall [44].
Although pre-treatment with GSNO yielded higher green fluorescence typical of Al in Al-sensitive roots (Table 1), this NO donor altered the Al distribution pattern in tissues preventing the massive accumulation of this metal cation in the vascular cylinder (Fig. 2). Because root sections were freehand prepared, it cannot be ruled out that the higher amounts of Al in GSNO-pre-treated Al-sensitive roots is because the analyzed section may be thicker than those derived from roots solely exposed to Al.
Incubation of wheat roots with NO precursors increased the NO levels in Al-sensitive roots but not in Al-tolerant ones which can be explained by the fact that Al-tolerant roots have much higher endogenous NO levels than those of Al-sensitive ones (Table 1). The endogenous NO seems to be sufficient to prevent Al uptake and dispersal in Al-tolerant root tissues, although one needs to consider that the supposedly NO excess, provided as NO3−, Arg or GSNO, may also be diverted to the S-nitrosylation of proteins or L-tyrosine nitration, events shown to take place in Citrus aurantium plants under drought [45]. An increment in NO levels was found to play a crucial role in the initiation of a tolerance response to Al stress in wheat, in which the NO response was Al-specific and much faster in the Al-tolerant cultivar (Jian-864) than the Al-sensitive one (Yang-5) [24].
The nitrate reductase (NR), whose substrate is NO3−, has been directly or indirectly implicated in the NO biosynthesis in plant cells [17], [18], [46]. The fact that Al did not interfere with NR activity in rose mallow [13] suggests that supplementation of Al-sensitive wheat roots (Anahuac) with the NR substrate may provide enough NO (Table 1) to inhibit Al uptake (Fig. 1). Similarly, leaves of an NR-defective Arabidopsis mutant infiltrated with Arg exhibited an increased amount of NO when compared with non-infiltrated leaves [18]. The pathway by which Arg leads to NO formation is still under investigation. Experimental evidence suggests that polyamines produced from arginase activity are metabolized to provide NO via unknown mechanisms [30], [47]. Also, the increased arginase activity in the leaves of tobacco plants upon salt stress was accompanied by the accumulation of higher amounts of NO [48].
The pattern of NO accumulation in roots was quite distinct between the cultivars, in which the metabolism of Al-tolerant roots favored NO accumulation in tissues that function as primary or secondary barriers to Al entry into vascular cylinder (endodermal and pericycle cells). The Al-triggered alteration of NO accumulation in tissues of Al-tolerant roots in direct contact with the metal cation is likely a strategy to prevent the stressing agent access to the inner cortex and vascular cylinder. In fact, prevalence of Al was recorded in the endodermis (only) of Al-tolerant wheat roots with no detectable green fluorescence registered in inner tissues (Fig. 1, Fig. 2). Alteration of NO distribution in different zones of Arabidopsis roots was reported elsewhere [3] and the data presented here confirm that the response pattern vary in wheat roots according to the degree of Al tolerance.
The negative effect of Al on the basipetal polar auxin transport in the epidermis and outer cortex cells and on signaling in roots has been shown in Arabidopsis plants [3], [49], in which AUX1 and PIN, proteins involved in IAA influx and efflux, respectively, were primarily Al targets [3], [8]. In addition, the role of auxins in modulating root architecture and the involvement of NO in root growth and development and in gravitropic response is known [50]. The crosstalk between auxins and NO appears to take place at all levels (synthesis, transport and perception) during early plant development [50]. However, the possible role of NO in the prevention of Al-induced IAA flow blockage and the anatomical extent of IAA flow reestablishment deserves more investigation. Herein, it is demonstrated that pre-treatment of wheat roots with NO precursors or an NO donor attenuated the inhibitory effect of Al on IAA flow towards root apices, independent of the cultivar. The reestablishment of IAA flow in the procambium and fundamental meristem cells (Fig. 5, Fig. 6), known to present AUX1 proteins [51], indicates that both acropetal and basipetal auxin transports were occurring in root apices pre-treated with NO precursors or an NO donor. Remarkably, exogenous NO applied in conjunction with an inhibitor of IAA transport guaranteed the presence of IAA in the roots of both cultivars even in the presence of Al (Fig. 5; Table 2), endorsing that NO from NO3− or Arg alleviated the Al-induced impairment of IAA flow in root cells. The much lower endogenous production of NO in Al-sensitive roots after Al treatment in comparison to Al-tolerant roots (Fig. 3; Table 1) partly explains the findings of Camargo et al. [52] in which Al-sensitive plants (Anahuac), but not Al-tolerant ones (BH1146), have their root growth affected by Al [52]. The Al effect on an Al-sensitive maize line was comparable to that of auxin transport inhibitors as morphogenic alterations took place in the Al-treated root apex [6]. Additionally, the polar auxin transport was found to be insensitive to Al in an Al-tolerant tobacco mutant (AlRes4) as such plants presented significant increase of actin production and microfilament packing upon Al treatment [53]. Nitrosylation of cytoskeleton proteins by NO [54] is a post-translational modification that may influence endocytosis and vesicle movement. Considering that IAA exportation is of secretory nature, as observed for neurotransmitters, it is proposed that endosome and vesicle recycling are essential to the polar transport of IAA [55]. Thus, it is likely that the NO that originated from NO3− or Arg or was released from GSNO could restore the movement of IAA-containing endosomes inhibited by Al, especially in root cells of the elongation zone.
Surprisingly, the treatment with Al decreased the LOOH levels in roots of both cultivars, a result that was more prominent in Al-tolerant roots (Table 2), opposing various reports for different plant species [10], [41], [56], [57]. Investigations carried out with maize plants, however, showed that Al did not affect the LOOH levels in roots [58], [59]. On the other hand, a decrease in LOOH levels was observed in Citrus sinensis (orange) culture cells exposed to Al, a phenomenon attributed to the increased activity of SOD, CAT and APX [60]. The results herein presented suggest that roots of wheat cv. BH1146 (Al-tolerant) decrease LOOH levels upon Al stress by boosting the antioxidant enzyme system as it occurs in orange culture cells. Furthermore, lipids did not seem to be the primary cellular target of Al-induced oxidative stress [59]. Further decreases of LOOH levels occurred in Al-treated roots pre-treated with NO precursors or an NO donor, indicating the protective role of NO in the cell. The NO was also shown to react with LOO• and/or alkoxyl radicals (LO•), dismantling LOOH formation [61]. The GSNO-caused S-nitrosylation of monodehydroascorbate reductase (antioxidant enzyme) stimulated the activity of this enzyme in Arabidopsis in response to Pseudomonas syringae pv. tomato [62]. Moreover, it cannot be excluded a possible increment of other antioxidant molecules as previously observed in wheat roots in response to Al [63]. The unchanged levels of oxidized proteins in Al-treated wheat supports the findings previously reported [64].
The rate of metabolization of NO precursors in Al-sensitive and Al-tolerant wheat roots may be different in both cultivars, explaining the distinct effects of NO3− and Arg on the activity of antioxidant enzymes in such plants (Table 4). Some Al-tolerant wheat cultivars have shown significantly higher NR activity than the Al-sensitive cultivar have in the first three hours of exposure to Al [24]. In this sense, it is possible that the stimulation of antioxidant enzymes by NO3− pre-treatment took place much earlier than the 48 h of Al exposure, the period in which samples were analyzed for the activity of antioxidant enzymes. The antioxidant role of putrescine, an Arg-derived polyamine, has been shown to partly attenuate Al toxic effects on wheat cv. Xi Aimai-1 [65]. Here, the higher levels of NO found in Al-tolerant wheat roots exposed to Al may contribute to the control of oxidative burst in the seedlings either by de novo synthesis or by activation of antioxidant enzymes.
Conclusions
Overall, the results demonstrate that NO, either generated from GSNO or produced from the metabolism of NO3− or Arg, alleviates the negative effects of Al on auxin flow. The higher endogenous NO levels in roots of Al-tolerant seedlings and their ability to further increase NO content upon Al stress may partly explain the innate tolerance of the wheat cv. BH1146 to one of the most widely distributed metals in soils.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
The authors are thankful to Laci Adolfo (University of North Texas, USA) for critical reading of the manuscript and Janaina S. Garcia and Advanio I. Siqueira-Silva (UFMG, MG, Brazil) for assistance with acquisition of freehand cross-sections and samples preparation for IAA immunolocalization, respectively. This work was supported by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). ROF was granted a graduate scholarship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and LVM is supported by a CNPq research fellowship.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Kochian L.V., Piñeros M.A., Hoekenga O.A. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil. 2005;274:175–195. [Google Scholar]
- 2.Aggarwal A., Ezaki B., Munjal A., Tripathi B.N. Physiology and biochemistry of aluminum toxicity and tolerance in crops. In: Tripathi B.N., Müller M., editors. Stress responses in plants. Springer International Publishing; Switzerland: 2015. pp. 35–37. [Google Scholar]
- 3.lléš P., Schlicht M., Pavlovkin J., Lichtscheidl I., Baluška F., Ovecka M. Aluminium toxicity in plants: internalisation of aluminium into cells of the transition zone in Arabidopsis root apices relates to changes in plasma membrane potential, endosomal behaviour, and nitric oxide production. J Exp Bot. 2006;57:4201–4213. doi: 10.1093/jxb/erl197. [DOI] [PubMed] [Google Scholar]
- 4.Wang L., Fan X.W., Pan J.L., Huang Z.B., Li Y.Z. Physiologicalcharacterization of maize tolerance to low dose of aluminum, highlighted by promotedleaf growth. Planta. 2015;242(6):1391–1403. doi: 10.1007/s00425-015-2376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peixoto P.H.P., Pimenta D.S., Cambraia J. Alterações morfológicas e acúmulo de compostos fenólicos em plantas de sorgo sob estresse de alumínio. Bragantia. 2007;66(1):17–25. [Google Scholar]
- 6.Doncheva S., Amenós M., Poschenrieder C., Barceló J. Root cell patterning: a primary target for aluminium toxicity in maize. J Exp Bot. 2005;56:1213–1220. doi: 10.1093/jxb/eri115. [DOI] [PubMed] [Google Scholar]
- 7.Tabaldi L.A., Nicoloso F.T., Castro G.Y., Cargnelutti D., Gonçalves J.F., Rauber R. Physiological and oxidative stress responses of four potato clones to aluminum in nutrient solution. Braz J Plant Physiol. 2007;19(3):211–222. [Google Scholar]
- 8.Kollmeier M., Felle H.H., Horst W.J. Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminum. Plant Physiol. 2000;122:945–956. doi: 10.1104/pp.122.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen H., Hou N.Y., Schlicht M., Wan Y.L., Mancuso S., Baluska F. Aluminium toxicity targets PIN2 in Arabidopsis root apices: effects on PIN2 endocytosis, vesicular recycling, and polar auxin transport. Chin Sci Bull. 2008;53:2480–2487. [Google Scholar]
- 10.Giannakoula A., Yupsanis T. Aluminum stress induces up-regulation of an efficient antioxidant system in the Al-tolerant maize line but not in the Al-sensitive line. Environ Exp Bot. 2010;67:487–494. [Google Scholar]
- 11.Liu Q., Yang J.L., He L.S., Li Y.Y., Zheng S.J. Effect of aluminum on cell wall, plasma membrane, antioxidants and root elongation in triticale. Biol Plant. 2008;52:87–92. [Google Scholar]
- 12.Jones D.L., Kochian L.V. Aluminum inhibition of the inositol 1,4,5-trisphosphate signal transduction pathway in wheat roots: a role in aluminum toxicity? Plant Cell. 1995;7:1913–1922. doi: 10.1105/tpc.7.11.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian Q., Sun D., Zhao M., Zhang W. Inhibition of nitric oxide synthase (NOS) underlies aluminum-induced inhibition of root elongation in Hibiscus moscheutos. New Phytol. 2007;174:322–331. doi: 10.1111/j.1469-8137.2007.02005.x. [DOI] [PubMed] [Google Scholar]
- 14.Ali B., Hasan S., Hayat S., Hayat Q., Yadav S., Fariduddin Q. A role for brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiata L Wilczek) Env Exp Bot. 2008;62:153–159. [Google Scholar]
- 15.Delledonne M., Xia Y.J., Dixon R.A., Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- 16.Clark D., Durner J., Navarre D.A., Klessig D.F. Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Mol Plant Microbe Interact. 2000;13:1380–1384. doi: 10.1094/MPMI.2000.13.12.1380. [DOI] [PubMed] [Google Scholar]
- 17.Modolo L.V., Augusto O., Almeida I.M.G., Magalhaes J.R., Salgado I. Nitrite as the major source of nitric oxide production by Arabidopsis thaliana in response to Pseudomonas syringae. FEBS Lett. 2005;579:3814–3820. doi: 10.1016/j.febslet.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 18.Modolo L.V., Augusto O., Almeida I.M.G., Pinto-Maglio C.A.F., Oliveira H.C., Seligman K. Decreased arginine and nitrite levels in nitrate reductase-deficient Arabidopsis thaliana plants impair nitric oxide synthesis and the hypersensitive response to Pseudomonas syringae. Plant Sci. 2006;171:34–60. [Google Scholar]
- 19.Arasimowicz M., Floryszak-Wieczorek J. Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant Sci. 2007;172:876–887. [Google Scholar]
- 20.Wilson I.D., Neill S.J., Hancock J.T. Nitric oxide synthesis and signalling in plants. Plant, Cell Environ. 2008;31:622–631. doi: 10.1111/j.1365-3040.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- 21.Moreau M., Lindermayr C., Durner J., Klessig D.F. NO synthesis and signaling in plants – where do we stand? Physiol Plant. 2010;138:372–383. doi: 10.1111/j.1399-3054.2009.01308.x. [DOI] [PubMed] [Google Scholar]
- 22.Sírová J., Sedlářová M., Piterková J., Luhová L., Petřivalský M. The role of nitric oxide in the germination of plant seeds and pollen. Plant Sci. 2011;181:560–572. doi: 10.1016/j.plantsci.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Wang H.-H., Huang J.-J., Bi Y.-R. Nitrate reductase-dependent nitric oxide production is involved in aluminum tolerance in red kidney bean roots. Plant Sci. 2010;179:281–288. [Google Scholar]
- 24.Sun C., Lu L., Liu L., Liu W., Yu Y., Liu X. Nitrate reductase-mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticum aestivum) New Phytol. 2014;201:1240–1250. doi: 10.1111/nph.12597. [DOI] [PubMed] [Google Scholar]
- 25.Wang H., Huang J., Liang W., Liang X., Bi Y. Involvement of putrescine and nitric oxide in aluminum tolerance by modulating citrate secretion from roots of red kidney bean. Plant Soil. 2013;366:479–490. [Google Scholar]
- 26.Wang Y.-S., Yang Z.-M. Oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant CellPhysiol. 2005;46:1915–1923. doi: 10.1093/pcp/pci202. [DOI] [PubMed] [Google Scholar]
- 27.Xue Y.-J., Tao L., Yang Z.-M. Aluminum-induced cell wall peroxidase activity and lignin synthesis are differentially regulated by jasmonate and nitric oxide. J Agr Food Chem. 2008;56:9676–9684. doi: 10.1021/jf802001v. [DOI] [PubMed] [Google Scholar]
- 28.He H.-Y., He L.-F., Gu M.-H., Li X.-F. Nitric oxide improves aluminum tolerance by regulating hormonal equilibrium in the root apices of rye and wheat. Plant Sci. 2012;183:123–130. doi: 10.1016/j.plantsci.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Sun C., Liu L., Yu Y., Liu W., Lu L., Jin C. Nitric oxide alleviates aluminum-induced oxidative damage through regulating the ascorbate glutathione cycle in roots of wheat. J Integr Plant Biol. 2015;57:550–561. doi: 10.1111/jipb.12298. [DOI] [PubMed] [Google Scholar]
- 30.Rockel P., Strube F., Rockel A., Wildt J., Kaiser W.M. Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot. 2002;53:103–110. [PubMed] [Google Scholar]
- 31.Tun N.N., Santa-Catarina C., Begum T., Silveira V., Handro W., Floh E.I. Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant CellPhysiol. 2006;47:346–354. doi: 10.1093/pcp/pci252. [DOI] [PubMed] [Google Scholar]
- 32.Kinraide T.B. Assessing the Rhizotoxicity of the Aluminate Ion, Al(OH)4−. Plant Physiol. 1990;94:1620–1625. doi: 10.1104/pp.93.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hem J.B., Roberson C.E. Form and stability of aluminium hydroxide complexes in dilute solution. Geol Survey Water Supply Pap. 1967;1827(A):55 pp. [Google Scholar]
- 34.Tice K.R., Parker D.R., Demason D.A. Operationally defined apoplastic and symplastic aluminum fractions in root tips of aluminum-intoxicated wheat. Plant Physiol. 1992;100:309–318. doi: 10.1104/pp.100.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moctezuma E. Changes in auxin patterns in developing gynophores of the peanut plant (Arachis hypogaea L.) Ann Bot. 1999;83(3):235–242. doi: 10.1006/anbo.1998.0814. [DOI] [PubMed] [Google Scholar]
- 36.Thomas C., Bronner R., Molinier E.P., van Onckelen H., Hahne G. Immuno-cytochemical localization of indole-3-acetic acid during induction of somatic embryogenesis in cultured sunflower embryos. Planta. 2002;215:577–583. doi: 10.1007/s00425-002-0791-8. [DOI] [PubMed] [Google Scholar]
- 37.Bedetti C.S., Jorge N.C., Trigueiro F.C.G., Bragança G.P., Modolo L.V., Isaias R.M.S. Detection of cytokinins and auxin in plant tissues using histochemistry and immunocytochemistry. Biotech Histochem. 2018;93(2):149–154. doi: 10.1080/10520295.2017.1417640. [DOI] [PubMed] [Google Scholar]
- 38.Karnovsky M.J. A formaldehyde-glutaraldehyde fixative on high osmolarity for use in electron microscopy. J Cell Biol. 1965;27:137–138. [Google Scholar]
- 39.Gay C.A., Gebicki J.M. Perchloric acid enhances sensitivity and reproducibility of the ferric-xylenol orange peroxide assay. Anal Biochem. 2002;304:42–46. doi: 10.1006/abio.2001.5566. [DOI] [PubMed] [Google Scholar]
- 40.Giannopolitis C.N., Ries S.K. Superoxide dismutases. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cakmak I., Horst J.H. Effects of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max) Physiol Plant. 1991;83:463–468. [Google Scholar]
- 42.Nakano Y., Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- 43.Bradford M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 44.Pan C.-L., Yao S.-C., Xiong W.-J., Luo S.-Z., Wang Y.-L., Wang A.-Q. Nitric oxide inhibits Al-induced programmed cell death in root tips of peanut (Arachis hypogaea L.) by affecting physiological properties of antioxidants systems and cell wall. Front Physiol. 2017;8:1037. doi: 10.3389/fphys.2017.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ziogas V., Tanou G., Belghazi M., Filippou P., Fotopoulos V., Grigorios D. Roles of sodium hydrosulfide and sodium nitroprusside as priming molecules during drought acclimation in citrus plants. Plant Mol Biol. 2015;89(4–5):433–450. doi: 10.1007/s11103-015-0379-x. [DOI] [PubMed] [Google Scholar]
- 46.Kolbert Z., Bartha B., Erdei L. Exogenous auxin-induced NO synthesis is nitrate reductase-associated in Arabidopsis thaliana root primordial. J Plant Physiol. 2008;165:967–975. doi: 10.1016/j.jplph.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 47.Groppa M.D., Rosales E.P., Iannone M.F., Benavides M.P. Nitric oxide, polyamines and Cd-induced phytotoxicity in wheat roots. Phytochemistry. 2008;69:2609–2615. doi: 10.1016/j.phytochem.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 48.da-Silva C.J., Fontes E.P.B., Modolo L.V. Salinity-induced accumulation of endogenous H2S and NO is associated with modulation of the antioxidant and redox defense systems in Nicotiana tabacum L. cv. Havana. Plant Sci. 2017;256:148–159. doi: 10.1016/j.plantsci.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 49.Sun P., Tian Q.-Y., Chen J., Zhang W.-H. Aluminium-induced inhibition of root elongation in Arabidopsis is mediated by ethylene and auxin. J Exp Bot. 2010;61:347–356. doi: 10.1093/jxb/erp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanz L., Albertos P., Mateos I., Sánchez-Vicente I., Lechón T., Fernández-Marcos M. Nitric oxide (NO) and phytohormones crosstalk during early plant development. J Exp Bot. 2015;66:2857–2868. doi: 10.1093/jxb/erv213. [DOI] [PubMed] [Google Scholar]
- 51.Swarup R., Friml J., Marchant A., Ljung K., Sandberg G., Palme K. Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev. 2001;15:2648–2653. doi: 10.1101/gad.210501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Camargo C.E.O., Santos R.R., Pettinelli-Júnior A. Trigo duro: tolerância à toxicidade do alumínio em soluções nutritivas e no solo. Bragantia. 1992;51:69–76. [Google Scholar]
- 53.Ahad A., Nick P. Actin is bundled in activation-tagged tobacco mutants that tolerate aluminum. Planta. 2007;225:451–468. doi: 10.1007/s00425-006-0359-0. [DOI] [PubMed] [Google Scholar]
- 54.Yemets A.I., Krasylenko Y.A., Lytvyn D.I., Sheremet Y.A., Blume Y.B. Nitric oxide signalling via cytoskeleton in plants. Plant Sci. 2011;181:545–554. doi: 10.1016/j.plantsci.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 55.Schlicht M., Strnad M., Scanlon M.J., Mancuso S., Hochholdinger F., Palme K. Auxin immunolocalization implicates vesicular neurotransmitter-like mode of polar auxin transport in root apices. Plant Signal Behav. 2006;1:122–133. doi: 10.4161/psb.1.3.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu F.J., Li G., Jin C.W., Liu W.J., Zhang S.S., Zhang Y.S. Aluminum-induced changes in reactive oxygen species accumulation, lipid peroxidation and antioxidant capacity in wheat root tips. Biol Plant. 2012;56:89–96. [Google Scholar]
- 57.Richards K.D., Schott E.J., Sharma Y.K., Davis K.R., Gardner R.C. Aluminum induces oxidative stress genes in Arabidopsis thaliana. Plant Physiol. 1998;1169:409–418. doi: 10.1104/pp.116.1.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giannakoula A., Moustakas M., Mylona P., Papadakis I., Yupsanis T. Aluminum tolerance in maize is correlated with increased levels of mineral nutrients, carbohydrates and proline, and decreased levels of lipid peroxidation and Al accumulation. J Plant Physiol. 2008;165:385–396. doi: 10.1016/j.jplph.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 59.Boscolo P.R.S., Menossi M., Jorge R.A. Aluminum-induced oxidative stress in maize. Phytochemistry. 2003;62:181–189. doi: 10.1016/s0031-9422(02)00491-0. [DOI] [PubMed] [Google Scholar]
- 60.Ghanati F., Morita A., Yokota H. Effects of aluminum on the growth of tea plant and activation of antioxidant system. Plant Soil. 2005;276:133–141. [Google Scholar]
- 61.Beligni M.V., Lamattina L. Nitric oxide interferes with plant photo-oxidative stress by detoxifying reactive oxygen species. Plant Cell Environ. 2002;25:737–748. [Google Scholar]
- 62.Romero-Puertas M.C., Campostrini N., Mattè A., Righetti P.G., Perazzolli M., Zolla L. Proteomic analysis of S-nitrosylated proteins in Arabidopsis thaliana undergoing hypersensitive response. Proteomics. 2008;8:1459–1469. doi: 10.1002/pmic.200700536. [DOI] [PubMed] [Google Scholar]
- 63.Hossain M.A., Hossain A.K.M.Z., Kihara T., Koyama H., Hara T. Aluminum-induced lipid peroxidation and lignin deposition are associated with an increase in H2O2 generation in wheat seedlings. Soil Sci Plant Nutr. 2005;61:223–230. [Google Scholar]
- 64.Kováčik J., Klejdus B., Hedbavny J. Effect of aluminium uptake on physiology, phenols and amino acids in Matricaria chamomilla plants. J Hazard Mater. 2010;178:949–955. doi: 10.1016/j.jhazmat.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 65.Yu Y., Jin C., Sun C., Wang J., Ye Y., Lu L. Elevation of arginine decarboxylase-dependent putrescine production enhances aluminum tolerance by decreasing aluminum retention in root cell walls of wheat. J Hazard Mater. 2015;299:280–288. doi: 10.1016/j.jhazmat.2015.06.038. [DOI] [PubMed] [Google Scholar]