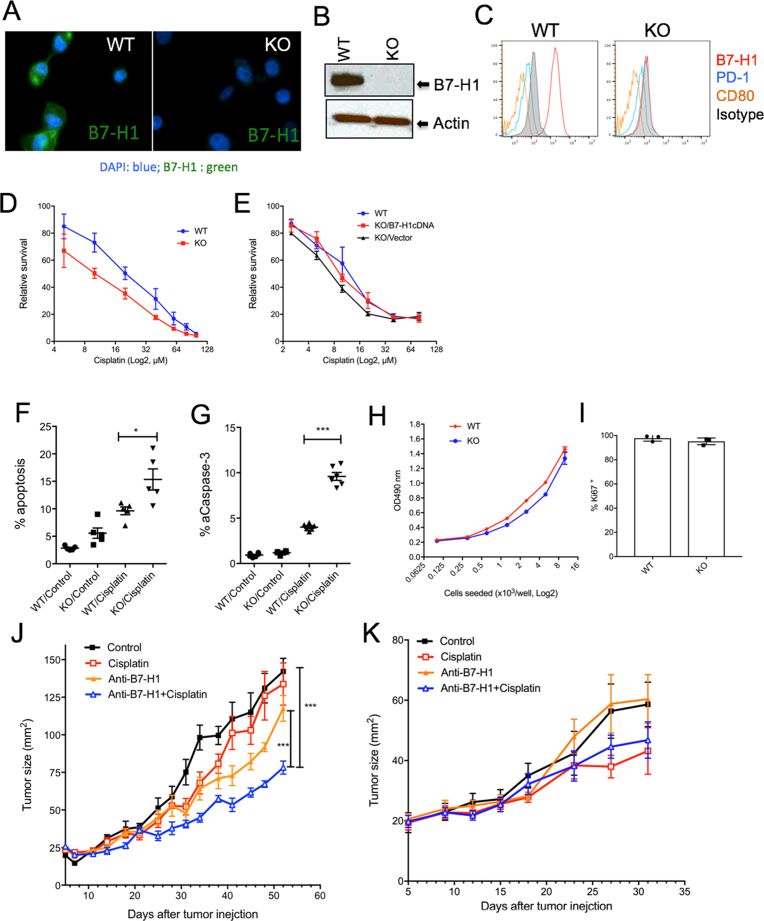
Fig. 2.
Targeting B7-H1 sensitized breast cancer cells to chemotherapy. B7-H1 KO MBA-MD-231 breast cancer cells were generated by CRISPR/Cas9 and confirmed by confocal microscopy (A), Western blotting (B) and flow cytometry (C). The absence of PD-1 and CD80 expression was confirmed in both wild type (WT) and B7-H1 KO cancer cells (C). The whole gel images of (B) were provided as the supplemental materials. (D) B7-H1 KO MBA-MD-231 cells are more sensitive to cisplatin than WT cells as determined by MTS assay. The p-value for area under the curve (dose-response curves) is significant (p < 0.01) in treatment with cisplatin. (E) Restored B7-H1 expression abolished the sensitivity of B7-H1 KO cancer cells to cisplatin. One of three experiments is shown. (F–G) Apoptosis of cancer cells treated with cisplatin (20 μM, 48 hr.) was analyzed by TMRE and Annexin V staining (F) and intracellular staining for active caspase-3 (G). Numbers of independent experiments (n = 5 in F, n = 6 in G). (H) MTS assay of the growth of B7-H1 WT and KO MDA-MB-231 breast cancer cells after 72 hours of culture. (I) Ki67 (a cell proliferation nuclear marker) expression by B7-H1 WT and KO MDA-MB-231 cells analyzed by flow cytometry. (J) WT MDA-MB-231 tumors or (K) B7-H1 KO MDA-MB-231 tumors were treated with B7-H1 antibody (Ab, H1A clone) or cisplatin, or both in vivo. ***P < 0.001 compared between B7-H1 Ab plus cisplatin and B7-H1 Ab along or control group as determined by Two-way ANOVA. One of two independent experiments is shown.