Abstract
Aims
Historically, cardiac resynchronization therapy (CRT) response in non‐left bundle branch block (non‐LBBB) patients has been suboptimal in comparison with that observed in left bundle branch block patients. The electrical activation pattern of the left ventricle (LV) is different between these two QRS morphologies. Small non‐randomized studies have suggested that targeting the LV wall with greatest electrical delay may be superior to conventional anatomical pacing from the lateral wall in non‐LBBB patients. This article outlines the design and rationale of a prospective, randomized, pilot study, which assesses the effect of a non‐traditional LV lead implant strategy on the clinical composite score after 12 months of follow‐up in a non‐LBBB patient population.
Methods
All patients will receive an Abbott quadripolar CRT‐D system (Quartet 1458Q LV lead with Unify Quadra™, Quadra Assura™ CRT‐D or any market‐approved CRT‐D device with quadripolar pacing capabilities). Patients will be randomized in a 2:1 ratio between a QLV‐based implant strategy vs. standard of care. Up to 250 patients will be enrolled in the study.
Conclusions
If the primary endpoint is achieved, this study will provide important information about reducing the non‐responder rate in non‐LBBB patients and provide further evidence for the QLV‐based implant strategy.
Keywords: QLV, Quartet, Non‐LBBB, RBBB, Implant strategy, Cardiac resynchronization therapy
Introduction
The goal of cardiac resynchronization therapy (CRT) is to electrically stimulate the site of the latest activation in the left ventricle (LV) sooner to synchronize ventricular contraction and improve cardiac output. Data from subgroup analyses1, 2, 3, 4 and meta‐analyses5, 6 of CRT trials report the relative absence of clinical benefit in patients with non‐left bundle branch block (non‐LBBB) vs. in those with left bundle branch block (LBBB). Potential reasons for this non‐response to CRT could be cardiac substrate differences, the absence of a significant electromechanical delay to be corrected by a device, and suboptimal placement of the LV lead. Several recent publications7, 8 have suggested that the conventional anatomical targeting of the lateral LV wall may not work as well in non‐LBBB, necessitating a more individualized electrical delay (QLV) targeting approach.
Studies have described the QLV interval as a measure of LV electrical delay and have shown it to correlate positively with acute (via LV dp/dtmax) and chronic (via clinical outcomes) responses to therapy.7, 8, 9, 10 The QLV interval is measured from the onset of the QRS wave in a surface electrocardiogram (ECG) to the first major deflection recorded from the electrode to be utilized for LV stimulation. The present study employs this interval as part of a non‐traditional implant strategy to assess the effect of LV lead location on the clinical composite score (CCS) after 12 months of follow‐up in a non‐LBBB patient population.
Study design
This is a prospective, double‐blinded, randomized, post‐market, pilot study conducted at up to 40 centres in the USA. Patients will be consented with an institutional review board‐approved consent form.
Major study inclusion/exclusion criteria
Eligible patients will have a non‐LBBB morphology, which includes complete right bundle branch block (RBBB), RBBB coupled with a fascicular delay, and interventricular conduction delay (IVCD) configurations ≥120 ms and have failed guideline‐directed medical therapy. Patients also must have a clinical indication for CRT per the 2013 updated American College of Cardiology Foundation (ACCF)/American Heart Association (AHA)/Heart Rhythm Society (HRS) guidelines [LV ejection fraction (LVEF) ≤ 35%, sinus rhythm, ischaemic or non‐ischaemic cardiomyopathy, and New York Heart Association (NYHA) class III/ambulatory class IV on guideline‐directed medical therapy]. Patients may receive a new CRT implant or undergo an upgrade from an existing implantable cardioverter defibrillator or pacemaker but must not have received greater than 10% right ventricular pacing.
Patients will be excluded if they have LBBB defined as QRS duration of >120 ms with predominantly negative QRS in lead V1 and upright, monophasic QRS in leads 1 and V6; incomplete RBBB; IVCD with a QRS duration between 110 and 119 ms; irreversible occlusion of venous access that will prevent placement of the CRT–implantable cardioverter defibrillator system either through the right or left upper extremity venous system; are undergoing LV lead placement via a surgical or epicardial approach; have cardiomyopathy due solely to valvular disease that is not repaired/replaced; have permanent atrial fibrillation; or are being upgraded primarily due to a high percent (>10%) of right ventricular pacing.
Enrolment
Patients will undergo screening evaluations as outlined by the inclusion/exclusion criteria. Demographic data such as the patient's gender, age, height, weight, ethnicity, race, cardiac disease history, arrhythmia history, smoking history, cardiac medications, indication for CRT‐D implant, NYHA class, and QRS duration will be collected at the enrolment visit. A report of the two‐dimensional (2D) echocardiogram will also be collected, and each patient will complete a Minnesota Living with Heart Failure (MLWHF) questionnaire. Randomization will be assigned up to 24 h prior to implant in a 2:1 ratio between the QLV and standard of care (SOC) implant strategy groups.
Implant
During the implant, the physician will utilize market‐approved product and technologies (Quartet™ 1458Q LV lead with Unify Quadra™, Quadra Assura™ CRT‐D or any market‐approved CRT‐D device with quadripolar pacing capabilities). Via the quadripolar lead, the electrophysiology (EP) recording system will be used to collect QLV measurements, here defined as the interval between earliest onset QRS (according to the limb lead that was determined to show the earliest onset) of the surface ECG to the centre of the largest peak, whether negative or positive, of the LV unipolar intracardiac electrogram during a cardiac cycle with the resolution of 5 ms (Figure 1 ).
Figure 1.
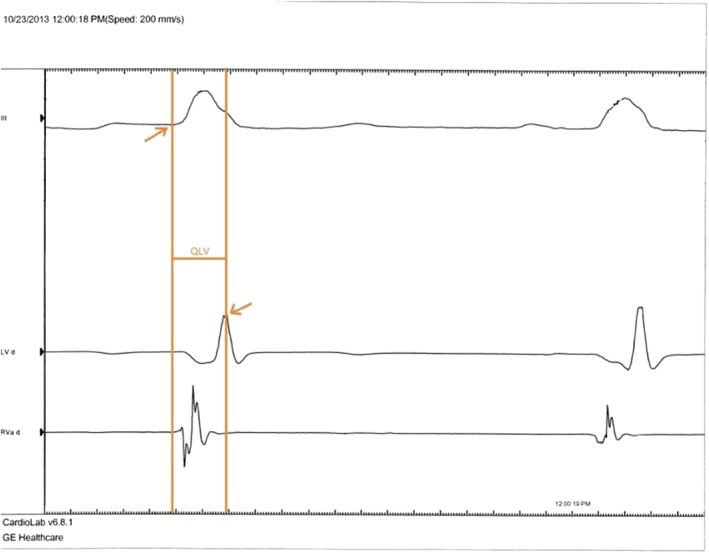
QLV measurement.
In the QLV randomization arm, the physician will assess at least two main branches of the coronary sinus for LV lead placement, testing first a non‐traditional vessel, inclusive of the anterior region, before testing a traditional free lateral branch. Upon initial placement, the physician will use the EP system to record and store snapshots, of at least 5 s duration, at each of the LV cathodes being tested. From these snapshots, the physician will measure the QLV interval and place the LV lead in the vein branch and cathode with the longest QLV measurement. The final paced vector will be programmed based on the cathode (electrode) selected. If there are multiple cathodes with relatively long QLV measurements, the physician will first choose the cathode with the longest QLV interval and test that cathode for phrenic nerve stimulation with a 10 V output. If that cathode has signs of positive phrenic nerve stimulation, the physician will move to the cathode that gives the second longest QLV. The physician will continue with the electrical testing until a cathode is found that does not have phrenic nerve stimulation. After implant, the QLV data from the EP system will be sent to a QLV core lab for evaluation.
In the SOC randomization arm, the LV lead placement will be conducted according to the physician's SOC implant approach, without the use of QLV measurements. The physician will also test the system at 10 V output to assess for phrenic nerve stimulation.
For subjects in both arms, capture threshold at 0.5 ms and lead impedance will be collected for the final programmed vector of the LV lead. Of note, all CRT implants require a venogram in both right anterior oblique and left anterior oblique at 20–40° views before the lead placement and a cine‐fluoroscopy in both right anterior oblique and left anterior oblique at the same angles after LV lead placement. The venograms and cine‐fluoroscopies will also be sent to a core lab for evaluation.
If an unsuccessful implant occurs, the physician may reattempt an endocardial implantation per his/her discretion. If a subject has an unsuccessful implant (e.g. a quadripolar LV lead is not implanted) and either a non‐transvenous approach for LV lead placement is planned or no reattempt is planned, the subject will be withdrawn from the study.
Follow‐up
Subjects who have a successful system implant will be seen pre‐discharge and at 3, 6, and 12 months post‐implant. At the pre‐discharge visit, all subjects will have a posterioranterior and lateral view chest X‐ray of the final lead position. During the follow‐up visits, subjects randomized to the QLV implant strategy will have QLV measurements completed with the programmer. The SOC subjects will have routine electrical measurements collected. For both groups, capture threshold at 0.5 ms and lead impedance will be collected for the final programmed vector of the LV lead. During the 6 and 12 month visits, a patient global assessment (PGA) and NYHA class assessment will be conducted by a cardiologist not involved in either the device implant procedure or device follow‐up. The subject will also have a 2D echocardiogram and complete a MLWHF questionnaire during the 6 and 12 month visits (Figure 2).
Figure 2.
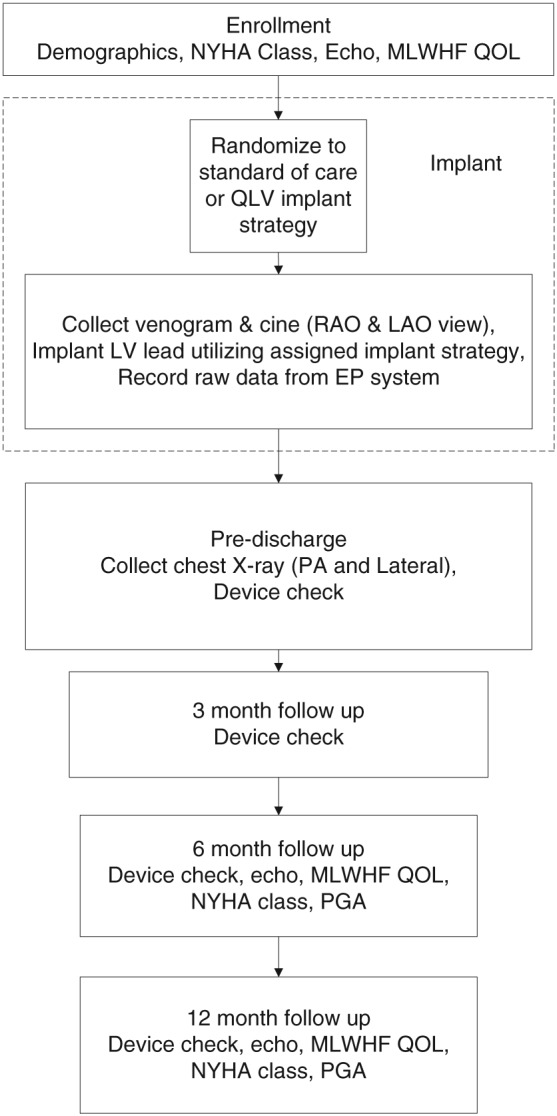
Study flowchart. LAO, left anterior oblique; LV, left ventricular; MLWHF, Minnesota Living with Heart Failure; NYHA, New York Heart Association; PA, posterioranterior; PGA, patient global assessment; RAO, right anterior oblique; QOL, quality of life.
Endpoint
The endpoint of this study is to evaluate CRT response of subjects in each arm via the CCS at 12 months using the decision algorithm described in Figure 3.11 This measure includes cardiovascular‐related mortality, heart failure (HF) hospitalizations, NYHA class, and PGA. Additional analyses will occur using demographics, QLV measurements from the QLV arm and QLV as a percentage of the QRS, QRS duration and morphology (RBBB, RBBB and left anterior fascicular block, RBBB and left posterior fascicular block, and non‐specific IVCD), MLWHF questionnaire scores, PGA, echocardiogram measurements [LVEF, LV end‐diastolic volume (LVEDV), LV end‐systolic volume (LVESV), and LVESV index], HF hospitalizations, total fluoroscopy time, mortality, and data from venous angiograms.
Figure 3.
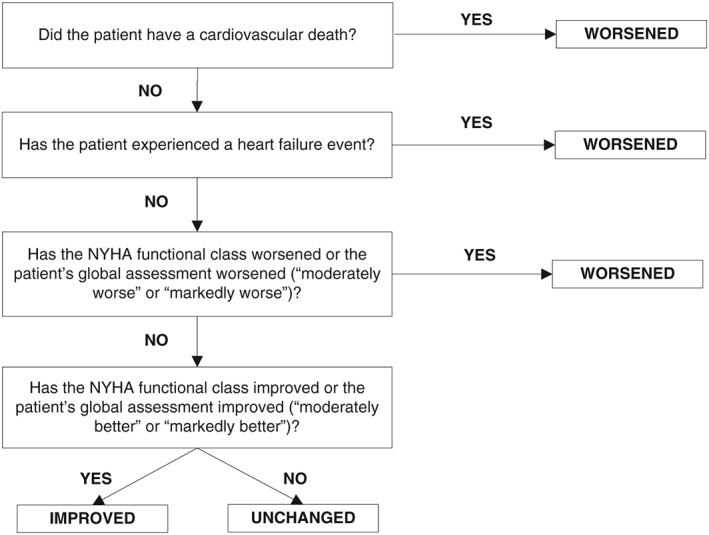
Decision algorithm to classify response to cardiac resynchronization therapy treatment. NYHA, New York Heart Association.
Assessments and adjudication
An independent Clinical Events Committee will adjudicate HF events, and an independent Mortality Committee will review and classify all patient deaths. The NYHA class assessment and PGA will be performed by a board‐certified cardiologist not involved in the device implant procedure nor in the patient's routine clinical care.
An LV lead placement core lab will evaluate implant venograms and pre‐discharge chest X‐rays to determine final placement of the quadripolar LV lead. A QLV core lab will evaluate the QLV measurements for each patient to determine accuracy of QLV measurement calculated by the implanting physician. Available electronic echocardiogram files from patients having a 2D echo completed during enrolment and 6 or 12 month visits will be sent to the echocardiogram core lab for review. The analysis of the echocardiogram will include LVEF (%), LV end‐diastolic diameter (mm), LVEDV (mL), LV end‐systolic diameter (mm), LVESV (mL), and LVESV index (mL/m2).
Blinding
The patients and cardiologists assessing NYHA class and PGA will be blinded to the randomization assignment. The Clinical Events and Mortality Committees will be blinded to patient and site identifiers but not to the patient's randomization assignment. Echocardiogram and QLV core labs will also not be blinded to the patient's randomization assignment.
Statistical analysis
This study was developed as a pilot study of 250 subjects who were randomized to the QLV and SOC implant strategy arms in a 2:1 ratio. Assuming a 23.5% attrition rate from enrolment to the 12 month follow‐up visit, 192 patients (128 in QLV and 64 in SOC) are expected to complete the follow‐up period.
Response in the SOC arm was predicted based on previously published results.4 Assuming 45.6% of the subjects in the SOC arm4 will improve clinically (CCS), the 192 patients could potentially provide an adequate power of approximately 80% to detect a 20% increase in the QLV group at the 5% significance level. Subjects will be analysed based on intention to treat. The study hypothesis will be tested at the 5% significance level using the one‐sided Fisher's exact test. The efficacy of the SOC vs. the QLV implant strategy on CRT response in the non‐LBBB population will be examined. Depending on the results, exploratory analyses may be performed on subgroups within the non‐LBBB population—including subgroups of RBBB and non‐RBBB, cardiomyopathy type, QRS duration, gender, and LVEF.
Discussion
Much of what is known about CRT is based on data from patients with LBBB because this is the predominant QRS morphology of patients meeting CRT indications. In fact, the majority of data on the impact of CRT in patients with non‐LBBB comes from retrospective and subgroup analyses vs. large randomized trials. Meta‐analyses5, 6 and a systematic review12 that include the largest trials studying CRT report that patients with non‐LBBB, including those with RBBB and with IVCD, do not receive clinical benefit from CRT as determined by soft or hard endpoints. Peterson et al.13 analysed Medicare claims data and similarly reported greater risks of adverse clinical outcomes, such as readmissions and complications, in non‐LBBB patients compared with LBBB patients with the same QRS duration.
However, there have been studies showing potential benefit of CRT in non‐LBBB patients if there is evidence of concomitant LV conduction delay. Electromechanical activation delay of the LV lateral wall was observed in patients with both types of block using a three‐dimensional non‐fluoroscopic mapping procedure14 and by tissue Doppler imaging.15 Hartlage et al. found that 45% of participants undergoing CRT implant had a type II LV wall motion pattern derived from cardiovascular magnetic resonance mapping as well as ECGs with either atypical LBBB or IVCD morphologies.16 Additionally, there were more responders to CRT who exhibited this heterogeneous U‐shaped activation pattern compared with non‐responders (78% vs. 40%, P = 0.038). The response rate for subjects with a type II pattern and LV lead placement near the latest contracting segment was significantly higher than that for subjects without both of these characteristics (61% vs. 7%, P = 0.003). Varma studied the inferolateral LV activation time in relation to QRS duration and morphology and found that patients with RBBB, as well as LBBB, morphology exhibited significantly longer LV activation time compared with the control group.17 These findings suggest the presence of substrate for CRT in non‐LBBB patients, though its location and composition may differ from that seen in LBBB patients.
In addition to assessing electromechanical delays with these methods, researchers have used the QLV interval to gauge electrical dyssynchrony. Zanon et al. showed how pacing at a site of maximum QLV interval corresponded to acute maximum increases in LV dP/dtmax in 31 of 32 patients undergoing CRT implant (13 of whom had a non‐LBBB configuration).7 Response rates to CRT at 6 months (as assessed by changes in LVESV, LVEDV, LVEF, and quality of life using scores on the MLWHF questionnaire) have also been shown to improve significantly in patients, regardless of QRS morphology, from the shortest to the longest quartile of QLV measurement in the final LV lead location.8 Researchers in this sub‐study also found that the median QLV value for the study (95 ms) was the optimal cut‐point for generating a statistically significant area under the receiver operating characteristic curve in the analyses of LVESV and quality of life endpoints. Moreover, two retrospective analyses including non‐LBBB patients showed significant longer term effects of QLV at 1 and 3 years on a composite endpoint of an increase in at least one NYHA class and/or LVEDV reduction ≥10%18 and time to first HF hospitalizations,19 respectively.
We believe that the present study is the first multicentre, prospective, randomized trial looking at the potential clinical impact of LV lead placement on CCS assessed at 12 months in non‐LBBB patients who have failed guideline‐directed medical therapy. Subjects are randomized prior to implant to determine whether the quadripolar LV lead of their CRT‐D device will be implanted via SOC per the implanting physician's discretion or via the QLV strategy as described. Investigators will explore, whether individualized implantation strategies using the QLV interval, as opposed to anatomical targeting of the LV lateral wall, can impact clinical outcome in this cohort of non‐LBBB patients. Study results will also provide data on longitudinal changes in clinical and echocardiographic measurements in non‐LBBB patients, which can serve as a baseline for future studies.
Conclusions
This pilot study will provide data on the effects of a QLV‐based implant strategy in the non‐LBBB patient population. Depending on the final results, this clinical trial will provide further insight on the role of the QLV‐based implant approach towards reducing the CRT non‐responder rate in this unique and under‐studied population.
Conflict of interest
Dr J.P.S. served as consultant to Abbott, Boston Scientific, Medtronic, LivaNova, Impulse Dynamics, Respicardia Inc., and Biotronik; Dr R.D.B. and Dr E.G.D. served as consultants to Abbott; Dr R.N.D. served as consultant to Abbott, Boston Scientific, and Zoll Medical; Dr M.L. served as consultant to Abbott, Boston Scientific, and Medtronic; Dr D.M. has nothing to disclose.
Funding
This study was supported by Abbott, Abbott Park, IL, USA.
Singh, J. P. , Berger, R. D. , Doshi, R. N. , Lloyd, M. , Moore, D. , Daoud, E. G. , and for the ENHANCE CRT Study Group (2018) Rationale and design for ENHANCE CRT: QLV implant strategy for non‐left bundle branch block patients. ESC Heart Failure, 5: 1184–1190. 10.1002/ehf2.12340.
Clinicaltrials.gov Identifier: NCT01983293.
References
- 1. Birnie DH, Ha A, Higginson L, Sidhu K, Green M, Philippon F, Thibault B, Wells G, Tang A. Impact of QRS morphology and duration on outcomes after cardiac resynchronization therapy results from the Resynchronization–Defibrillation for Ambulatory Heart Failure Trial (RAFT). Circ Heart Fail 2013; 6: 1190–1198. [DOI] [PubMed] [Google Scholar]
- 2. Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt S, Brown M, Cannom D, Daubert JP, Eldar M, Gold MR, Goldberger JJ, Goldenberg I, Lichstein E, Pitschner H, Rashtian M, Solomon S, Viskin S, Wang P, Moss AJ, on behalf of the MADIT‐CRT Investigators . Effectiveness of cardiac resynchronization therapy by QRS morphology in the Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy (MADIT‐CRT). Circulation 2011; 123: 1061–1072. [DOI] [PubMed] [Google Scholar]
- 3. Stockburger M, Moss AJ, Klein HU, Zareba W, Goldenberg I, Biton Y, McNitt S, Kutyifa V. Sustained clinical benefit of cardiac resynchronization therapy in non‐LBBB patients with prolonged PR‐interval: MADIT‐CRT long‐term follow‐up. Clin Res Cardiol 2016; 105: 944–952. [DOI] [PubMed] [Google Scholar]
- 4. Gold MR, Thébault C, Linde C, Abraham WT, Gerritse B, Ghio S, St John Sutton M, Daubert JC. The effect of QRS duration and morphology on cardiac resynchronization therapy outcomes in mild heart failure: results from the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) Study. Circulation 2012; 126; 822–829. [DOI] [PubMed] [Google Scholar]
- 5. Sipahi I, Chou JC, Hyden M, Rowland DY, Simon DI, Fang JC. Effect of QRS morphology on clinical event reduction with cardiac resynchronization therapy: meta‐analysis of randomized controlled trials. Am Heart J 2012; 163: 260–267. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cunnington C, Kwok CS, Satchithananda DK, Patwala A, Khan MA, Zaidi A, Ahmed FZ, Mamas MA. Cardiac resynchronisation therapy is not associated with a reduction in mortality or heart failure hospitalisation in patients with non‐left bundle branch block QRS morphology: meta‐analysis of randomised controlled trials. Heart 2015; 101: 1456–1462. [DOI] [PubMed] [Google Scholar]
- 7. Zanon F, Baracca E, Pastore G, Fraccaro C, Roncon L, Aggio S, Noventa F, Mazza A, Prinzen F. Determination of the longest intra‐patient left ventricular electrical delay may predict acute hemodynamic improvement in cardiac resynchronization therapy patients. Circ Arrhythm Electrophysiol 2014; 7: 377–383. [DOI] [PubMed] [Google Scholar]
- 8. Gold MR, Birgersdotter‐Green U, Singh JP, Ellenbogen KA, Yu Y, Meyer TE, Seth M, Tchou PJ. The relationship between ventricular electrical delay and left ventricular remodelling with cardiac resynchronization therapy. Eur Heart J 2011; 32: 2516–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh JP, Fan D, Heist EK, Alabiad CR, Taub C, Reddy V, Mansour M, Picard MH, Ruskin JN, Mela T. Left ventricular lead electrical delay predicts response to cardiac resynchronization therapy. Heart Rhythm 2006; 3: 1285–1292. [DOI] [PubMed] [Google Scholar]
- 10. Butter C, Auricchio A, Stellbrink C, Fleck E, Ding J, Yu Y, Huvelle E, Spinelli J; Pacing Therapy for Chronic Heart Failure II Study Group . Effect of resynchronization therapy stimulation site on the systolic function of heart failure patients. Circulation 2001; 104: 3026–3029. [DOI] [PubMed] [Google Scholar]
- 11. Packer M. Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail 2001; 7: 176–182. [DOI] [PubMed] [Google Scholar]
- 12. Nery PBHA, Keren A, Birnie DH. Cardiac resynchronization therapy in patients with left ventricular systolic dysfunction and right bundle branch block: a systematic review. Heart Rhythm 2011; 8: 1083–1087. [DOI] [PubMed] [Google Scholar]
- 13. Peterson PN, Greiner MA, Qualls LG, al‐Khatib SM, Curtis JP, Fonarow GC, Hammill SC, Heidenreich PA, Hammill BG, Piccini JP, Hernandez AF, Curtis LH, Masoudi FA. QRS duration, bundle‐branch block morphology, and outcomes among older patients with heart failure receiving cardiac resynchronization therapy. JAMA 2013; 310: 617–626. [DOI] [PubMed] [Google Scholar]
- 14. Fantoni C, Kawabata M, Massaro R, Regoli F, Raffa S, Arora V, Salerno‐Uriarte JA, Klein HU, Auricchio A. Right and left ventricular activation sequence in patients with heart failure and right bundle branch block: a detailed analysis using three‐dimensional non‐fluoroscopic electroanatomic mapping system. J Cardiovasc Electrophysiol 2005; 16: 112–119. [DOI] [PubMed] [Google Scholar]
- 15. Takamatsu H, Tada H, Okaniwa H, Toide H, Maruyama H, Higuchi R, Kaseno K, Naito S, Kurabayashi M, Oshima S, Taniguchi K. Right bundle branch block and impaired left ventricular function as evidence of a left ventricular conduction delay. Circ J 2008; 72: 120–126. [DOI] [PubMed] [Google Scholar]
- 16. Hartlage GR, Suever JD, Clement‐Guinaudeau S, Strickland PT, Ghasemzadeh N, Magrath RP, Parikh A, Lerakis S, Hoskins MH, Leon AR, Lloyd MS, Oshinski JN. Prediction of response to cardiac resynchronization therapy using left ventricular pacing lead position and cardiovascular magnetic resonance derived wall motion patterns: a prospective cohort study. J Cardiovasc Magn Reson 2015; 17: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Varma N. Left ventricular conduction delays and relation to QRS configuration in patients with left ventricular dysfunction. Am J Cardiol 2009; 103: 1578–1585. [DOI] [PubMed] [Google Scholar]
- 18. Polasek R, Kucera P, Nedbal P, Roubicek T, Belza T, Hanuliakova J, Horak D, Wichterle D, Kautzner J. Local electrogram delay recorded from left ventricular lead at implant predicts response to cardiac resynchronization therapy: retrospective study with 1 year follow up. BMC Cardiovasc Disord 2012; 12: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kandala J, Upadhyay GA, Altman RK, Parks KA, Orencole M, Mela T, Kevin Heist E, Singh JP. QRS morphology, left ventricular lead location, and clinical outcome in patients receiving cardiac resynchronization therapy. Eur Heart J 2013; 34: 2252–2262. [DOI] [PubMed] [Google Scholar]


