Abstract
In recent years, cochlear implants (CIs) have been provided in growing numbers to people with not only bilateral deafness but also to people with unilateral hearing loss, at times in order to alleviate tinnitus. This study presents audiological data from 15 adult participants (ages 48 ± 12 years) with single sided deafness. Results are presented from 9/15 adults, who received a CI (SSD-CI) in the deaf ear and were tested in Acoustic or Acoustic + CI hearing modes, and 6/15 adults who are planning to receive a CI, and were tested in the unilateral condition only. Testing included (1) audiometric measures of threshold, (2) speech understanding for CNC words and AzBIO sentences, (3) tinnitus handicap inventory, (4) sound localization with stationary sound sources, and (5) perceived auditory motion. Results showed that when listening to sentences in quiet, performance was excellent in the Acoustic and Acoustic + CI conditions. In noise, performance was similar between Acoustic and Acoustic + CI conditions in 4/6 participants tested, and slightly worse in the Acoustic + CI in 2/6 participants. In some cases, the CI provided reduced tinnitus handicap scores. When testing sound localization ability, the Acoustic + CI condition resulted in improved sound localization RMS error of 29.2° (SD: ±6.7°) compared to 56.6° (SD: ±16.5°) in the Acoustic-only condition. Preliminary results suggest that the perception of motion direction, whereby subjects are required to process and compare directional cues across multiple locations, is impaired when compared with that of normal hearing subjects.
Keywords: Single sided deafness, Cochlear implant, Tinnitus, Sound localization, Bilateral
1. Introduction
1.1. Cochlear implantation in single-sided deafness
Hearing loss affects over 300 million people worldwide, and over 300,000 patients have received a cochlear implant (CI) (Macherey and Carlyon, 2014; National Institutes of Health, 2016). Until 10—15 years ago, the standard of care had been to provide a single CI to patients with bilateral severe-to-profound hearing loss. However, several changes have been taking place in the criteria for implantation in one or both ears. For example, there has been a steady growth in adoption of bilateral implantation as standard clinical practice for these patients (Balkany et al., 2008; Lovett et al., 2015; Peters et al., 2010; Ramsden et al., 2009), although the effectiveness of the second CI continues to be studied in both children and adults (e.g., Litovsky et al., 2012; Wilson et al., 2011). A second example is a unique group of patients who have single-sided deafness (SSD) who receive a CI in the deaf ear, despite having normal hearing (NH) in the opposite ear (hence, referred to as SSD-CI). Although this configuration of hearing loss is not considered to be an indication for implantation in many countries, including the U.S., patients with SSD-CI are growing in number for a variety of reasons. Approximately 0.01—0.02% of the adult population is diagnosed with SSD annually, which when multiplied over the lifespan translates to ~1% of the population (Baguley et al., 2006). Treatment with a CI for SSD was first reported in 2008 (Van de Heyning et al., 2008) with more recent reports as well (e.g., Tokita et al., 2014; Zon et al., 2016), representing a recent shift in the clinical indications for intervention. Currently, SSD-CI is in an investigational stage in the U.S. (http://clinicaltrials.gov). Patients with unilateral deafness have not been traditionally treated with a CI, possibly because it has been assumed that NH in one ear provides sufficient auditory functionality. However, there is growing evidence to the contrary.
The motivation for adding a second ear in SSD-CI has been in some studies, rooted in the clinical notion that electrical stimulation reduces negative effects due to tinnitus (e.g., Arndt et al., 2011; Gartrell et al., 2014; Vermeire et al., 2008). In addition, there is motivation to help patients overcome deficits due to unilateral hearing loss, and ideally regain benefits that are known to occur in listeners with NH, including improvement in sound localization abilities and in speech understanding in noisy environments. However, the extent to which intervention with a CI ultimately results in NH-like performance remains to be determined. In October of 2017, an online search (www.ncbi.nlm.nih.gov/pubmed) using the PubMed search engine with key words ‘single sided deafness cochlear implant’ returned approximately 130 citations. Initial SSD-CI studies were aimed at tinnitus suppression, a condition that is extremely difficult to treat and can have major consequences on patients’ quality of life. In fact, there have been several reports of successful outcomes, whereby patients with SSD-CI showed reduction or complete suppression of tinnitus (Arndt et al., 2011; Buechner et al., 2010; Mertens et al., 2016). In addition, given that the SSD-CI meant that patients transitioned from having unilateral to bilateral hearing, studies also reported improved speech understanding in noise, improved sound localization and quality of life (Arndt et al., 2011; Bernstein et al., 2016; Vermeire and Van de Heyning, 2009; Zeitler et al., 2015). A recent review examining published studies related to SSD-CI found variability in outcomes: some studies demonstrated weak evidence of benefit whereas other studies showed encouraging effect of CI as a rehabilitative option for SSD (Cabral Junior et al., 2016).
1.2. Spatial hearing in SSD-CI
From an ecological standpoint, the ability to locate sounds helps with a range of important everyday activities, such as alerting an individual to approaching sources of danger, and directing attention for communication in noisy environments. In normal-hearing listeners, sound localization abilities on the horizontal plane are known to be mediated by the binaural system, whereby sounds that arrive from various locations in space reach the ears at different times and with different intensities (except for locations directly in front or behind), producing interaural differences in time (ITD) and level (ILD). The binaural system has exquisite sensitivity to these differences, and neural circuits in the brainstem are involved in processing the information (Grothe et al., 2010; Joris and Yin, 2007), so that ultimately the listener perceives sound source directionality (for reviews see Blauert, 1997; Middlebrooks and Green, 1991). Sound localization in individuals with SSD-CI is a particularly interesting phenomenon to investigate, because these listeners need to integrate acoustic and electric inputs across the ears to experience directional hearing. Studies on sound localization in CI users have, to date, primarily focused on individuals who are fitted with bilateral CIs, or with bimodal hearing, who use a CI in one ear and hearing aid in the opposite ear. Results from numerous studies on bilateral CI users have demonstrated that sound localization is generally better when bilateral CIs are used compared with situations in which only a single CI is used (Dunn et al., 2008; Grantham et al., 2007; Litovsky et al., 2009; Neuman et al., 2007; Nopp et al., 2004). Similar findings in bimodal patients suggest that the addition of a hearing aid to the ear contralateral to the CI results in improved sound localization performance in some but not all listeners, and benefits depend on best aided listening conditions (Dorman et al., 2016; Dunn et al., 2005; Firszt et al., 2018; Gifford et al., 2014; Veugen et al., 2016). In the bimodal population patients rely on integration of high-rate electric stimulation with variable-rate frequency dependent acoustic stimulation, yet the auditory system can, in some conditions, integrate information from the two different input modes such that spatial hearing is facilitated compared with conditions in which either ear is used in isolation. It must be added however, that in both bilateral CI and bimodal studies, patients typically perform worse than NH listeners, suggesting that the spatial hearing mechanisms in the patient groups are not capable of representing acoustic space with the same precision as a binaural acoustic system.
There are numerous explanations for what may be compromised in these patient groups. First, in people who experience prolonged auditory deprivation prior to receiving the CI, there is likely to be degeneration in neural pathways that mediate spatial sensitivity (Gordon et al., 2015; Tillein et al., 2016) and temporal processing in auditory cortex (Fallon et al., 2014). Second, there may be poor representation of binaural cues due to factors such as lack of deliberate synchronization of inputs in the two ears, mismatches in frequency-specific stimulation between the right and left ears (Kan et al., 2013; Long et al., 2003; Poon et al., 2009), or poor processing of temporal information at monaural levels (Ihlefeld et al., 2015). Third, there is lack of temporal fine structure in electric stimulation, which diminishes sensitivity to fine- structure ITDs and therefore compromises spatial hearing abilities (for reviews see Kan and Litovsky, 2015; van Hoesel, 2004). Although it is possible that ITDs in the envelopes of signals are present to some extent through CI processors, their utility remains to be demonstrated.
Table 1 shows a summary of seven studies published to date on sound localization in SSD-CI participants. Overall, adding the CI to a NH ear improved sound localization ability, though the reduction in localization error after the CI was added is similar to bilateral CI users, which is approximately 20—30°. A few important points are not apparent from prior studies, first, there is little comparison made before and after surgery, i.e., baseline unilateral performance followed by transition to the bilateral mode. Second, while overall errors are generally reported, the type of errors that are made are not clearly described relative to the normal vs. implanted ear. Third, while studies in stationary sound localization are informative, this situation is not necessarily representative of real-world listening. Sound sources in the real-world are not always stationary but can change locations dynamically. For example, a moving vehicle or a target talker needs to be successfully tracked to locate its position. In our lab, we have conducted preliminary experiments examining a listener’s ability to track a moving sound source. In this study, we include preliminary data from four SSD-CI participants who were tested on their ability to perceive moving sound sources when integrating their acoustic and electric inputs.
Table 1.
Previous sound localization studies in adults with SSD.
| Study | Subjects | Stimulus | Target Angular Range | Hearing Modality | Duration of CI use | Avg. RMS Error |
|---|---|---|---|---|---|---|
| Arndt et al. (2011) | 11 SSD adults | OLSA Sentences | −90° to +90° | NH only | 33.9° | |
| NH + CI | 6 mo | 15° | ||||
| Gartrell et al. (2014) | 1 SSD adult | 4-burst pink noise train | −90° to +90° | NH only | 29.68° | |
| CI only | 43.87° | |||||
| NH + CI | 6 mo | 30.21° | ||||
| NH only | 32.96° | |||||
| CI only | 66.7° | |||||
| NH + CI | 9 mo | 26.39° | ||||
| NH only | 28.76° | |||||
| CI only | 50.73° | |||||
| NH + CI | 12 mo | 23.23° | ||||
| Távora-Vieira et al. (2015) | 16 SSD adults | Narrow-band noise | −60° to +60° | NH only | 48.9° | |
| NH + CI | 6–18 mo | 22.8° | ||||
| Zeitler et al. (2015) | 45 NH adults | Wide-band noise | −90° to +90° | 6° | ||
| 27 BiCI adults | 29° | |||||
| 9 SSD adults | NH + CI | 2–33 mo | 30° | |||
| Grossmann et al. (2016) | 12 SSD adults | Speech-shaped noise | −90° to +90° | NH only | 63.2° | |
| NH + CI | 8–53 | 27.6° | ||||
| Rahne and Plontke (2016) | 10 SSD adults | White noise | −90° to +90° | NH + CI | 1 mo | 52° |
| NH + CI | 3 mo | 46° | ||||
| NH + CI | 6 mo | 39° | ||||
| NH + CI | 12 mo | 32° | ||||
| Dillon et al. (2017) | 20 SSD adults | Speech-shaped noise | −90° to +90° | NH + CI | 1 mo | 37° |
| NH + CI | 3 mo | 27.67° | ||||
| NH + CI | 6 mo | 27° | ||||
SSD = single sided deafness.
NH = normal hearing.
CI = cochlear implant.
RMS = root mean square.
To date, studies on auditory motion in NH adults have focused on measuring minimum audible angle (MAA) and minimal audible movement angle (MAMA). For instance, in Harris and Sergeant (1971) auditory motion detection was investigated by employing a loudspeaker placed on a cart which was pulled by an apparatus to elicit perception of motion. MAA was measured in binaural and monaural conditions, MAA was lower in the binaural condition, suggesting binaural hearing is necessary for good motion detection. In Grantham (1986) listeners discriminated stationary from moving sound sources, and threshold was defined as the MAMA, which was reported to be ~5°. Perrott and Marlborough (1989) showed that continuous sound source motion leads to lower MAMA compared to discrete stationary noise bursts, hence, a continuously moving sound source provided a benefit. Based on these studies in NH adults, we selected a paradigm in which auditory motion is simulated by a continuously moving sound source.
This paper provides the first set of data from a group of SSD-CI participants who are enrolled in studies in our lab. We report on the speech understanding and spatial hearing abilities of 15 adult participants. As described below, some participants have already received a CI, and performance was compared for the conditions when listening with the Acoustic ear alone vs. the Acoustic + CI condition. Other participants have been tested only in their SSD condition prior to receiving their CI, providing their own control for pre-implantation as a benchmark to be for future comparison with their Acoustic + CI condition. In one set of measures, we used standard audiological measures of speech understanding for words and sentences. In a second experiment, we studied sound localization for stationary sounds. And in the third experiment for which we only have data from 4 participants, we measured auditory motion perception to utilize a paradigm that has more realistic connection to the auditory environment in which listeners spend much of their time.
2. Method
2.1. Participants
Table 2 shows demographic information of the 15 participants in this study who were in the CI group; 9/15 participants had undergone CI surgery and were tested after device activation. MAG was implanted with a Hybrid L24 which is a short electrode, array but did not use electric-acoustic stimulation, as she lost her residual hearing after receiving the CI. The nine participants reside in different parts of the US and are seen at different clinics. Thus, device programming techniques may have varied from subject to subject. Similarly, rehabilitation strategies, if provided, may have varied. In addition, 6/15 participants are enrolled in a clinical study, such that they are tested before and after activation of the CI. These six participants provided data for the pre-Cl stage only. All of these 15 participants traveled to Madison Wl for the research, and were in the lab for approximately two days of testing, with frequent breaks.
Table 2.
Demographic Subject Information. SSNHL stands for Sudden Sensorineural Hearing Loss. *duration of deafness up to the date of testing for subjects who have not yet received a Cl.
| Subject | Sex | Etiology of deafness | Affected | Age at onset of | Length of time between deafness and | Use of Hearing Devices | Internal | CI | CI Experience |
|---|---|---|---|---|---|---|---|---|---|
| Code | Ear | deafness | CI activation (yr;mo) | CI | Processor | (yr; mo) | |||
| MAA | M | Head trauma | R | 51 | 3;11 | unknown | Nucleus CI512 |
Nucleus 5 |
0;9 |
| MAB | M | Meniere’s Disease, progressive loss |
R | 36 | 7;0 | BAHA for 2 yrs; 3 mo | MedEl | OPUS2 | 0;11 |
| MAC | F | Meningioma and surgical complications |
R | 46 | 7;0 | none | AB HR90K | Harmony 2;3 | |
| MAE | M | SSNHL | R | 61 | 0;10 | none | Nucleus CI512 |
Nucleus 5 |
4;4 |
| MAF | F | SSNHL | L | 27 | 0;2 | none | Nucleus CI24RE |
Nucleus 6 |
2;3 |
| MAG | F | SSNHL | R | 60 | 1;8 | none | Hybrid L24 |
Nucleus 6 |
0;9 |
| MAH | M | SSNHL | L | 37 | 1;6 | none | MedEl Flex 24 |
MedEl Rondo |
1;8 |
| MAI | M | SSNHL | L | 54 | 21;0 | none | Nucleus CI24RE |
Nucleus 5 |
4;0 |
| MAJ | M | SSNHL | L | 38 | 2;6 | CROS for 2yrs; 6mos | AB HR90K | Naida CI Q70 |
1;0 |
| MBA | F | Temporal bone fracture | R | 44 | 3;3* | SoundBitefor 18 months |
NA | ||
| MBB | M | SSNHL | R | 40 | 6;7* | none | NA | ||
| MBC | F | SSNHL | R | 66 | 0;11* | none | NA | ||
| MBD | M | SSNHL | R | 46 | 1;10* | HAfor6 months; BAHA for 6 months |
NA | ||
| MBE | F | SSNHL | R | 25 | 1;4* | CROS for 3—4 months | NA | ||
| MBF | F | SSNHL | L | 43 | 1;7* | none | NA | ||
In terms of the spatial hearing measures, the 9 participants who had already received a Cl were tested in a traditional sound local-ization paradigm. Due to time constraints, only 4 of these partici-pants were additionally tested in an auditory motion perception task. To provide a benchmark for the auditory motion perception task, 10 NH participants (2 male, 8 female, mean age = 23.4 years) were also tested. All NH participants passed audiometric testing at octave frequencies from 250 to 8000 Hz, and their hearing thresholds were less than or equal to 20 dB HL. The NH participants were students at the University of Wisconsin-Madison and were paid for their participation. Spatial hearing tests were conducted in a single-walled sound booth while audiological measures were conducted in a standard double-walled sound booth (lAC). Experimental protocols were within standards set by the National ln- stitutes of Health and approved by the University of Wisconsin- Madison’s Human Subjects Institutional Review board.
2.2. Audiological evaluation
Audiological evaluation was conducted as part of the study protocol and consisted of pure-tone thresholds, monosyllabic word recognition (CNC) testing in quiet, and sentences (AzBlO) tested in quiet and noise. On the latter, speech stimuli were presented at 65 dB SPL, and the background noise consisted of the AzBlO clinical test which has 4-talker babble and was presented at a signal to noise ratio (SNR) of 0 dB. Stimuli in the Acoustic ear condition were presented through insert phones (ER3). ln the Cl condition, stimuli were presented in free field from a loudspeaker positioned in front at a distance of 1 m. ln addition, in the Cl condition the sound in the NH ear was attenuated by inserting an ear plug and placing an ear muff over the ear. The effective attenuation of the muffled NH ear was assessed by measuring thresholds for the Acoustic ear while plugged and with the CI turned off.
In addition, participants completed the Tinnitus Handicap Inventory (THI). Participants who were part of the clinical study and tested prior to receiving a CI completed audiological evaluation with their acoustic ear only, and the THI was administered with reference to having hearing in only one ear. Participants who were tested after receiving a CI (SSD-CI) completed the audiological evaluation in three conditions: NH ear only (Acoustic), implanted ear only with acoustic ear muffled (CI), and with both NH and CI ears together (Acoustic + CI). When tested after receiving the CI, the THI questionnaire was administered twice, once in reference to when they are wearing the CI and again in reference to when the CI is off (e.g., at bedtime or when first waking in the morning).
2.3. Spatial hearing: stationary and dynamic sound source localization
2.3.1. Localization of stationary sound sources
To date, we have measured the spatial hearing abilities of nine SSD-CI participants who have had experience with their CI for at least nine months. These participants were tested in two conditions: Unilateral (Acoustic ear only) and Bilateral (Acoustic + CI).
Stationary sound localization abilities were measured in a soundproof booth. The study had begun during the time when the lab was using an array of 19 loudspeakers spaced 10° apart (−90° to +90° ) in the frontal horizontal plane, at a sound level of 50 dB (with a rove of ± 4 dB SPL), hence two of the participants were tested with that configuration. Later, an additional 18 loudspeakers were added to the array in order to simulate moving sound sources. In order to allow sufficient time for the battery of tests, the remaining participants were tested at only 13 loudspeaker locations that were spaced 15° apart. In addition, the stimulus presentation levels were increased to 60 dB (with a rove of ± 4 dB SPL) to more closely match conditions used in audiological measures. In one case (MAJ) testing was conducted at 50 dB SPL with the 13 loudspeakers, due to an error during testing that was only discovered later. Rather than omitting the data, they are included here. In either loudspeaker array configuration, an acoustically transparent curtain was used to conceal all loudspeakers from the subjects. On each trial, the stimulus consisted of a train of 4 pink noise bursts each 170 ms in duration with 10 ms on/off ramp and an interstimulus interval of 50 ms. Participants were tested on 15 trials per target location tested in blocks of 5 repetitions. Each block of Acoustic and Acoustic + CI conditions were tested in random order. The task was to identify the location of a target sound source on a graphical user interface (GUI) that had a pictorial representation of a continuous loud-speaker array along the azimuthal plane. Further details about the setup and test procedures can be found in previous studies using this sound localization task and configuration (e.g., Gartrell et al., 2014; Jones et al., 2014). The results are reported for individual participants, and the effect of adding the CI is quantified as the improvement in root mean squared (RMS) localization error between the Acoustic and Acoustic + CI conditions. Further, an analysis of localization performance was conducted to examine biases in localization responses when listening with and without the CI.
2.3.2. Perception of auditory motion
Auditory motion perception ability was assessed in a free-field experiment which used an array of 37 loudspeakers spaced 5° apart in the front-half horizontal plane. Moving sounds were simulated using vector based amplitude panning (Pulkki, 1997). Stimuli were 500 ms, bandlimited (150—6000 Hz) white noise tokens that moved in an arc of radius 1.2 m and angular ranges of 0° (i.e., stationary), 10°, 20°, and 40°. Moving stimuli always ended at one of 19 target loudspeaker locations that were spaced 10° apart, (−90° to +90° in azimuth). The listeners initiated presentation of each stimulus by pressing on a button on a touch screen. After the stimulus was presented, the listener indicated their response on an arc on the touch screen that represented the loudspeaker array. Listeners could provide two response types: 1) a single press to indicate a stationary sound was perceived; 2) a solid line drawn within the arc to indicate the direction and distance of a moving sound. Stimuli were presented 10 times for a stationary sound and 10 times in each of the moving sound source conditions (5 presentations left-moving and 5 presentations right-moving), from the 19 target locations mentioned previously. Thus, the stationary condition had 190 trials, the 10° condition had 360 trials, the 20° condition had 340 trials, and the 40° condition had 300 trials, for a total of 1190 trials. All angular range conditions were randomized throughout each testing session, and testing was conducted in blocks that contained approximately 60 trials each, with the testing session lasting around 2 hours per participant. The data collected for auditory motion perception were analyzed using three different measures: 1) The accuracy with which a moving sound could be distinguished from a stationary sound; 2) The accuracy in reporting the direction of the sound when the trial was correctly identified as moving; and 3) The ability to track how far a moving sound source traversed the horizontal plane.
3. Results
3.1. Audiological results
Fig. 1A shows pure-tone thresholds for the Acoustic ear only for all 15 SSD participants. All participants had normal to near-normal thresholds at test frequencies ≤4000 Hz in their Acoustic ear. Fig. 1B shows the effective attenuation obtained with the ear plug plus ear muff, assessed by measuring thresholds for the Acoustic ear while muffled and with the CI turned off.
Fig. 1.
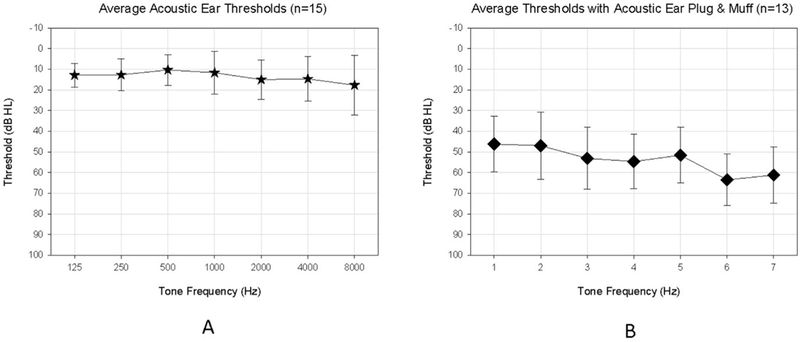
Panel A shows pure-tone thresholds (mean ± standard deviation) for the Acoustic ear only for the participants with single-sided deafness (SSD), and Panel B shows the effective attenuation obtained with the ear plug plus ear muff, assessed by measuring thresholds for the Acoustic ear while muffled and with the cochlear implant (CI) turned off.
Fig. 2 has nine panels which show individual thresholds for the SSD-CI participants tested to date, comparing the Acoustic + CI and CI conditions. While displaying large individual variability, most SSD-CI participants had thresholds in the CI condition that were in the mild hearing loss range for the mid-frequency regions, suggesting good audibility with the CI.
Fig. 2.
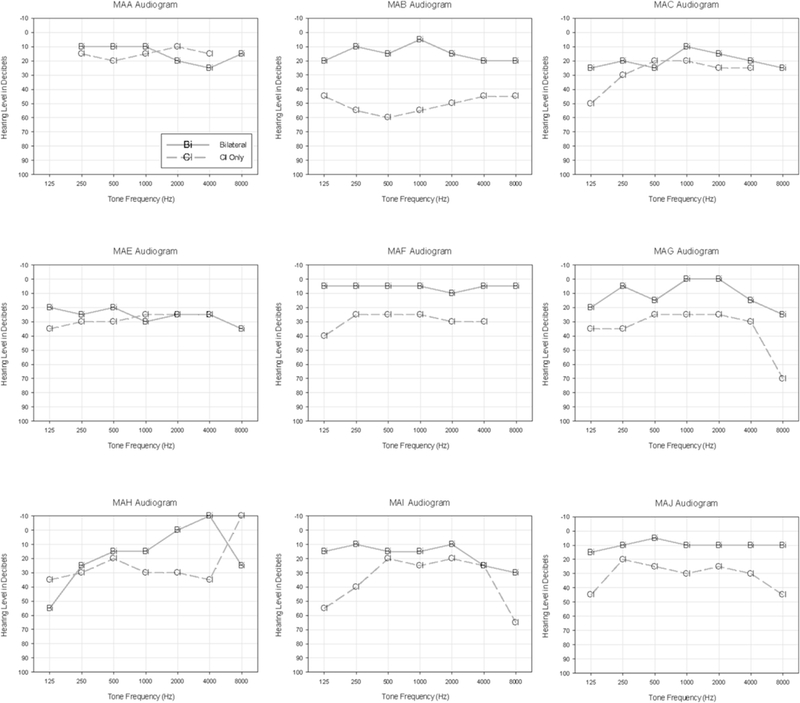
In each of the 9 panels there are individual thresholds for participants with single-sided deafness and a CI (SSD-CI). Acoustic + CI and CI conditions are compared.
Fig. 3 (panels A and B, respectively) shows speech test results for CNC words and phonemes in quiet, comparing percent correct scores in three conditions: Acoustic, Acoustic + CI, and CI. All participants had excellent scores when listening with the Acoustic ear alone in quiet and with the Acoustic + CI ears, obtaining CNC word scores of 96.7 ± 3.2% and 96.5 ± 3.0%, respectively. This finding suggests that the added CI did not interfere with performance in quiet.
Fig. 3.
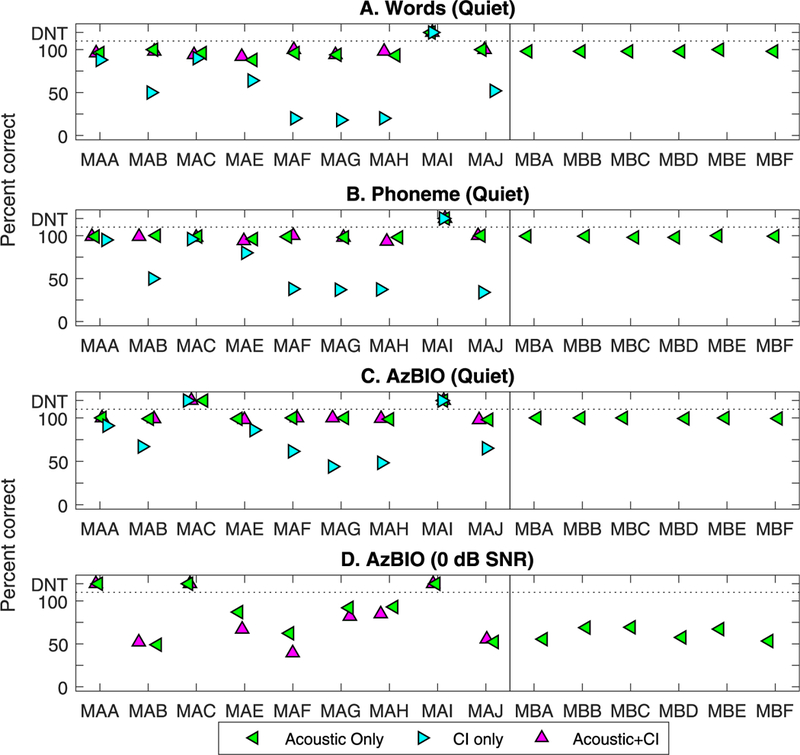
Percent correct scores from the CNC words testing, including words (top) and phonemes (bottom), comparing Acoustic, cochlear implant (CI) and Acoustic + CI. Along the xaxis are individual subjects, with the single-sided deafness and a CI (SSD-CI) subjects on the left and SSD subjects on the right, hence only the Acoustic data point for the latter. Results are shown for CNC words (panel A), phonemes (panel B), AZBio in quiet (panel C), and AzBio in noise (panel D). Percent correct scores shown in different symbols are compared in three conditions: Acoustic, Acoustic + CI, and CI.
The results from the AzBIO testing in quiet and in noise are shown in Fig. 3 (panels C and D, respectively. Left-most panels show data for the 9 SSD-CI participants and right-most panels show data for the 6 pre-Cl participants. In the former, due to time constraints, we were able to test 7 participants in quiet and 6 participants in noise. ln quiet, all participants achieved ceiling scores in the Acoustic and Acoustic + Cl conditions. In the condition with 0 dB SNR noise, the Acoustic condition was tested in all 15 participants, and the Acoustic + Cl condition was tested in the post-Cl group, to evaluate the outcome of adding the Cl to the hearing ear. Considerable variability was seen among the Acoustic ear only condition. Results for the Acoustic + Cl condition shows that 4 participants (MAB, MAG, MAH and MAJ) obtained scores within 6.12 ± 3.52% of their Acoustic only scores, while 2 participants performed slightly worse with the added Cl (MAE decreased by 20.0% and MAF decreased by 22.9%).
Fig. 4 shows results from the THl; 2/9 SSD-Cl subjects (those who already had the Cl at the time of testing) did not report having any tinnitus at all, thus their scores on the THl = 0. The remaining 7/ 9 SSD-Cl subjects completed the THl at the time of testing. We asked them to complete the questionnaire twice. One time they were asked to complete the THl during Cl use (i.e., most of the day). The other time, they were asked to complete the THl in relation to when they have the Cl off (i.e., at bedtime, while sleeping, or first thing in the morning). The data suggest that 4/7 SSD-Cl subjects reported reduced tinnitus when wearing the Cl compared to when the Cl was turned off. Two other subjects reported a very slight decrease and one subject reported a small increase in THl. Finally, 6 SSD subjects (pre-Cl) only completed the THl once during this interval.
Fig. 4.
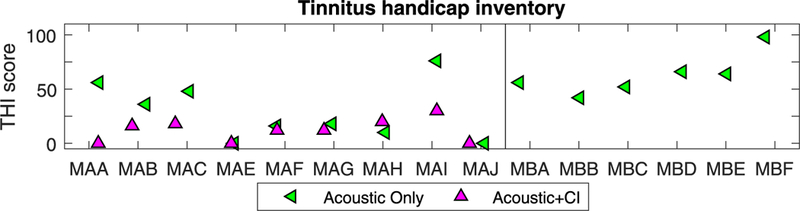
Results from the Tinnitus Handicap Inventory are shown for all individual participants. Along the x-axis are individual subjects; those with single-sided deafness and a CI (SSD-CI) are on the left and those with SSD are on the right, hence only the Acoustic data point for the latter.
3.2. Localization of stationary and perceptually moving sound sources
Fig. 5 shows the sound localization responses of the 9 participants who had received the Cl, comparing performance in the Acoustic condition (left panels) with the Acoustic + Cl (Bilateral) condition (right panels). The localization responses were binned at 5° intervals as a function of the source location (shown in grey). Next to the subject code, the ear that had acoustic hearing is shown in parentheses. In the Acoustic only condition, some SSD-CI listeners (MAB, MAG, MAH, and MAI) typically responded towards their acoustic ear. On the other hand, there are some SSD-CI listeners (MAA, MAC, MAE, MAF, MAJ) that can localize some targets contralateral to their acoustic ear when listening in the Acoustic only condition. These trends are more clearly seen in the mean localization responses (black squares).
Fig. 5.
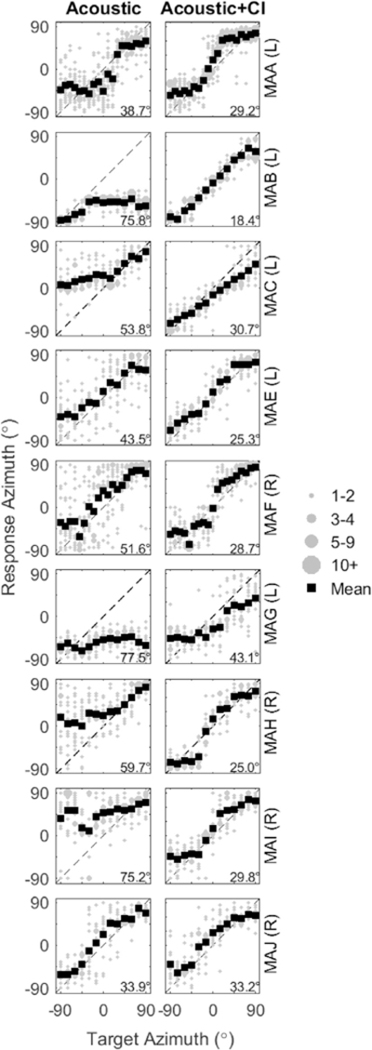
Sound localization responses of the 9 subjects with single-sided deafness and a cochlear implant (CI) (SSD-CI) are shown in two conditions: Acoustic (left panels) and Acoustic + CI (right panels). Each panel represents data from one subject in one condition, such that responses are plotted as a function of target angle from −90° to +90°. Responses were binned at 5_ intervals as a function of the source location, and the size of the symbol represents the number of responses at that location. Averages of the responses are shown in black filled squares. Root mean square (RMS) values for each listener are reported in the top left of each plot. The diagonal dashed lines represent the line of unity along which correct responses would appear. Next to the subject code, the ear that had acoustic hearing is shown in parentheses.
Fig. 6 shows the RMS errors for the Acoustic and Acoustic + CI conditions along with data from Jones et al. (2014). Most SSD-CI listeners showed an improvement (lower RMS errors) when the CI was added to the acoustic ear, with the exception of MAJ who showed relatively good localization in both conditions. Specifically, in the Acoustic + CI condition, the average RMS error was 29.26° (SD: ±6.72°); this constitutes an average improvement in RMS error by 27° compared to the Acoustic condition. When compared to the localization performance of bilateral CI and NH listeners fromJones et al., RMS errors of SSD-CI listeners are within the range of errors seen in bilateral CI users, and were generally greater than errors seen in NH listeners.
Fig. 6.
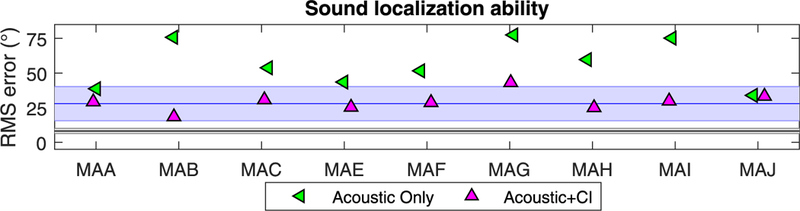
Root mean square (RMS) localization error values are shown for each participant when listening in the Acoustic and Acoustic + CI conditions, for sound source locations varying from −90° to +90° in 10° steps. For comparison, data fromJones et al. (2014) are included; RMS errors for NH and bilateral CI participants are shown in the black and green regions, respectively. The shaded areas show the standard deviations and the lines show the group means..
Even though sound localization is an important component of spatial hearing, there is a more complex task that involves the perception of a moving sound source. In this study, we obtained preliminary data on 4 SSD-CI participants using the task that evaluates perceived auditory motion. Fig. 7 and Table 3 show the proportion of trials on which the sound source was moving, and reported as moving. Two of the SSD-CI subjects (MAH, MAJ) showed correct movement identification in the same range as NH listeners, and in fact MAH showed NH-like performance at all angular ranges tested. One SSD-CI subject (MAF) correctly identified a sound source as moving in the NH range for two of the four angular range conditions (10° and 20°), and subject MAG rarely reported the sound source as moving.
Fig. 7.
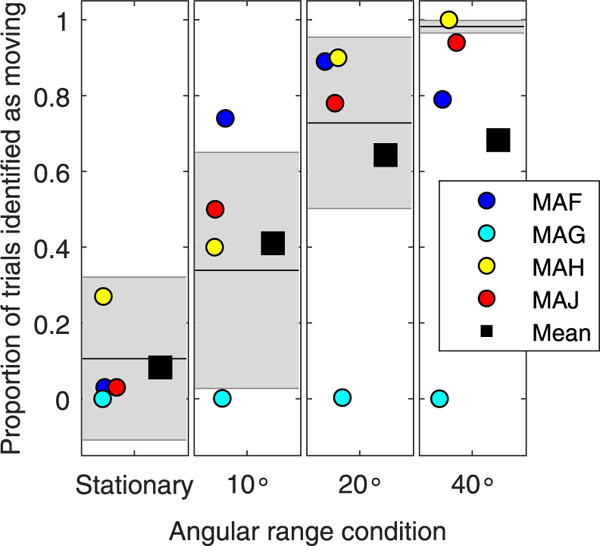
Proportion of trials on which the sound source was moving, and reported as moving, is shown for each of the angular range conditions tested (stationary, 10°, 20°, 40°). The individual data points represent 4 different listeners with single-sided deafness and a cochlear implant (CI) (SSD-CI), and the mean is shown as the square symbol. The normal hearing (NH) mean and standard deviation are shown in the black lines and shaded grey areas, respectively.
Table 3.
The calculated proportion of trials reported as moving are reported for each subject, in each of the angular range conditions.
| Subject Id | Stationary | 10° | 20° | 40° |
|---|---|---|---|---|
| MAF | 5/190 = 0.02 | 268/360 = 0.74 | 303/340 = 0.89 | 238/300 = 0.79 |
| MAGa | 0/185 = 0.00 | 1/349 = 0.002 | 1/328 = 0.003 | 0/292 = 0.00 |
| MAH | 69/190 = 0.36 | 144/360 = 0.40 | 306/340 =0.90 | 300/300 = 1.0 |
| MAJ | 4/190 = 0.021 | 180/360 = 0.50 | 268/340 = 0.78 | 284/300=0.94 |
Testing for Subject MAG was limited due to time constraints.
While motion detection may be possible in an Acoustic + CI hearing condition, the ability to identify the direction of motion is much poorer than that of NH listeners. The results from Fig. 8, while preliminary, suggest that performance was near chance or slightly above, hence direction per se is challenging for these listeners. Note that subject MAG was removed from Fig. 8 due to poor performance in the previously mentioned task. Results from Fig. 9 focus on the ability to track how far a moving sound source traversed the horizontal plane. In NH listeners, the 10°, 20° and 40° range conditions yielded average reported ranges of 19.88° ( ±3.37°), 23.92° ( ±3.3°) and 33.12 ( ±6.52°, respectively, suggesting a tendency to over-estimate the range of movement in the 10° range, and to under-estimate in the 40° range. One SSD-CI listener (MAH) always over-estimated the range of motion. SSD-CI listener MAJ had results that were within the NH range for all conditions. Subject MAF over-estimated how far the sound source moved for the two smaller- range conditions, but had data within the NH range for the larger range condition.
Fig. 8.
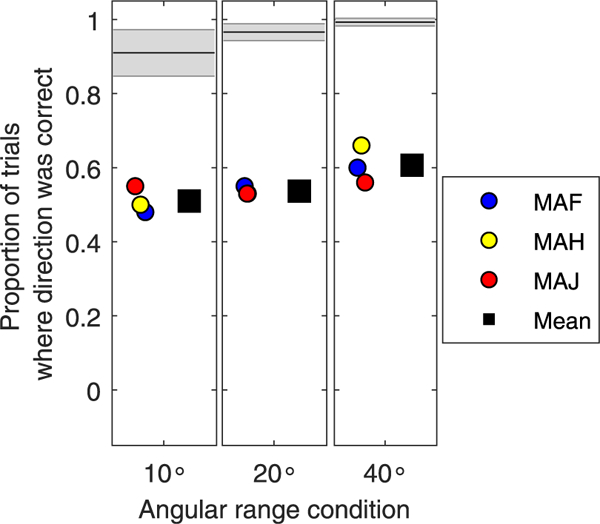
Proportion of trials on which the sound source was reported as moving and the direction of movement was correct, is shown for each angular range condition tested (10_, 20_, 40_). The individual data points represent the 3 listeners with single-sided deafness and a cochlear implant (CI) (SSD-CI), who had heard sounds as moving, and the mean is shown as the square symbol. The normal hearing (NH) mean and standard deviation are shown in the black lines and shaded grey areas, respectively.
Fig. 9.
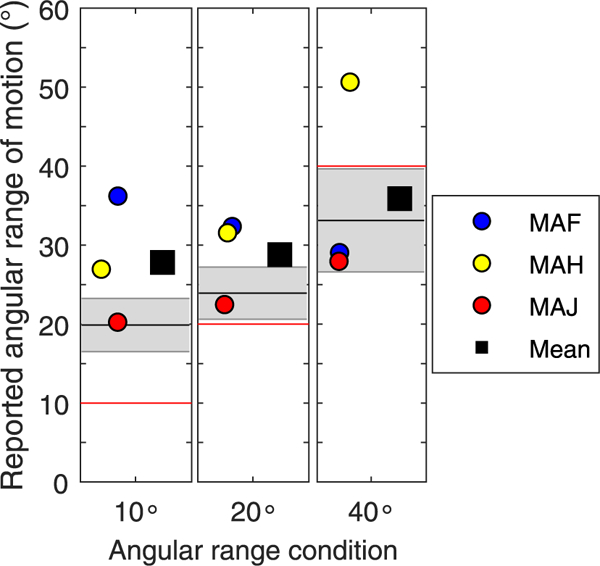
The ability to track a moving sound source traversing the horizontal plane, is shown for each angular range condition tested (10°, 20°, 40°). The individual data points represent the 3 SSD-CI listeners who were able to track the moving sounds, and the mean is shown as the square symbol. The NH mean and standard deviation are shown in the black lines and shaded grey areas, respectively. A red line is shown in each angular range condition to represent the target angular range of the moving sound source that was presented. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
In most listening environments people are surrounded by multiple sound sources that can have similarity in content and voice characteristics. These multiple sources can also arrive from different locations, or from locations nearby one another. The auditory system manages this cacophony of inputs through several mechanisms, including binaural mechanisms that provide information regarding source location. For NH listeners, ITDs and ILDs play an important role in helping people determine source locations. As described above, some patients with SSD received a CI and in many cases tinnitus suppression was an important motivating factor for surgery. However, not all SSD CI users reported tinnitus preoperatively. An additional benefit to cochlear implantation in SSD patients is the potential restoration of spatial hearing abilities.
First, our audiological data showed that when listening to sentences in noise at 0 dB SNR, there appears to be either no change or slightly worse percent correct speech understanding (Fig. 4). While we cannot rule out factors associated with device fitting strategies and rehabilitation strategies differing between subjects, the audi- ological data suggest that for some of the participants the degraded auditory input provided by the CI may not have integrated optimally with the acoustic input of the NH ear. This type of finding has been reported in patients who use a CI in one ear and a hearing aid in the contralateral ear (e.g., Crew et al., 2015; Litovsky et al., 2006), although in one study the addition of a CI in an ear contralateral to a hearing aid did not have negative impact on speech scores (Mok et al., 2006). In a vocoder study, Fu et al. (2017) recently found that integration of high frequency electric and low frequency acoustic hearing is better when the two types of stimuli are presented to the same ear compared with when they are presented into opposite ears, though the addition of low frequency acoustic hearing in either ear was always better than listening to the CI alone. However, the poorer performance associated with adding acoustic hearing to the contralateral ear in these studies may be due to limited low frequency acoustic audibility. One reason for poor integration across the ears could be mis-matched inputs across from the acoustic ear with electric ear. Prior studies (e.g., Fowler et al., 2016; Guerit et al., 2014; Wess et al., 2017) show that there are two types of mismatch that can affect performance: (1) a mismatch in the frequency inputs across the ears, and (2) a temporal mismatch between the stimulation from the CI and the acoustic input in the contralateral ear. Further, abnormally broad spectral fusion in hearing-impaired listening may also underlie the poorer performance (Reiss et al., 2016).
A second result from our study suggests that a CI can, in some cases, provide reduced tinnitus handicap reports, especially for the 4 participants with the highest tinnitus handicap in the Acoustic condition. The effectiveness of electrical stimulation on tinnitus suppression has been reported previously (Arndt et al., 2011; Arts et al., 2012; Vermeire et al., 2008). One caveat to the tinnitus measure is that it is not an objective measure of a condition but rather a subjective report, and may be difficult to tease apart from other subjective medical concerns. Finally, in the present study, not all patients with unilateral deafness have tinnitus as a comorbidity, suggesting that SSD-CI as a clinical treatment option might become viable for alleviating not only tinnitus but limitations due to poor spatial hearing abilities (Carlson et al., 2018; Doge et al., 2017; Van de Heyning et al., 2016).
The third set of measures in the current study, and the primary set of data, focused on the extent to which the addition of a CI to the Acoustic condition resulted in improved sound localization ability. Data from nine participants suggest that the second auditory input through electrical stimulation can improve sound localization by an average of ~30°; in one case there was no improvement, and in the eight other participants improvements ranged from ~10° to almost 60° (see Fig. 9). Thus, the relatively degraded and spectrally simplified signal provided by the CI is capable of adding sufficient information to the auditory system to facilitate improvement in sound localization. The improvement is very similar to the improvement seen in bilateral CI users. For example, in Litovsky et al. (2009), RMS errors in sound localization when listening with a unilateral CI compared to bilateral CIs, dropped from 58.5°±15.2°—28.8°±12.5°. In this study, our SSD-CI listeners RMS errors improved from 56.630±16.580 —29.26°±6.72°, which is highly comparable. In addition, the RMS errors of individuals with SSD-CI in this study are comparable to previous findings on sound localization ability of individuals with SSD-CI (see Table 1).
It must be noted however, that the improvement typically measured in adult subjects does not result in localization performance that is similar to that seen in NH listeners (e.g., Grantham et al., 2007; Jones et al., 2014; Litovsky et al., 2009; Nopp et al., 2004). The argument for the gap in performance between NH and bilateral CI users has been that with two CIs, the auditory system lacks access to localization cues that relyon temporal fine structure, that bilateral CI users may have poor neural survival at some places of stimulation along one or both cochlear arrays, and that mismatched stimulation across the ears can render binaural cues to be poorly represented (for review see Kan and Litovsky, 2015). In SSD-CI patients, the factors that limit sound localization can be similar to factors in bilateral CI users. For example, the lack of acoustic hearing in one ear means that the temporal fine structure cues required for processing ITDs at low frequencies are poor or absent. In addition, SSD-CI users might have ‘holes in hearing’ and poor neural survival in the deaf ear, as well as a CI programmed with degraded inputs that are not well matched to the acoustic information in the NH ear (Firszt et al., 2018; Vermeire and Van de Heyning, 2009; for a recent review see Cabral Junior et al., 2016). Some attempts to simulate aspects of SSD-CI with a vocoder that presented low frequency information to the Acoustic condition, which is different from actual SSD-CI, have shown degraded performance (e.g., Fu et al., 2017). SSD-CI patients also differ from bilateral CI users, in that they are likely to have a full compliment of highly functioning hair cells in the NH ear. Hence, if good temporal fine structure cues can be delivered to any region of the electrode array with fidelity, that would likely enhance localization performance without the need to match stimulation with fidelity across the two ears as has been shown in bilateral CI users (e.g., Long et al., 2003; Kan et al., 2013; Poon et al., 2009).
Finally, in this study we began to investigate an aspect of spatial hearing that has the potential to reveal aspects of real-world functioning not revealed by traditional sound localization of stationary sources. We presented preliminary data from 4 SSD-CI participants, showing that the proportion of trials on which the sound source was reported as moving was within the range of NH listeners for 2/4 participants. However, the actual source direction was poorly perceived. The auditory motion task thus captures an important aspect of listening in everyday situations, with implications for environmental safety. We had begun this preliminary investigation to ascertain whether normal acoustic input integrated with electrical stimulation from a CI would be adequate to perceive a moving sound source. In fact, in some cases the opposite seems to be true, at least in the SSD-CI participants tested to date, whereby, when subjects are required to process and compare directional cues across multiple locations, performance was impaired despite wearing the CI in addition to the acoustic ear. This might be partially due to independent inputs from the CI device and acoustic ear, which may result in poor integration of spatial cues across the ears. For example, poor integration may occur if the CI has automatic gain control to prevent sounds from being too intense, but which at the same time introduces compression in the loudness growth, which is likely to contribute to mis-matched dynamic ranges in the two ears and thus poor representation of ILD cues. Further research on this topic might reveal whether additional experience with the CI, possibly with multisensory training regarding spatial locations, could enhance performance in realistic listening situations (e.g., Isaiah et al., 2014). Finally, the participants tested here only had one to two years of listening with their CI and it is possible that additional experience would improve performance.
This study has a few limitations that should be noted, in the event they can be overcome with future studies. For example, the number of subjects was somewhat low, and the inter-subject variability in outcomes was high, thus we are not in a position to draw definitive conclusions about the functional role of adding a CI to a NH ear in SSD patients. The fact that subjects were fitted with different types of devices, and within devices there were not consistent fitting strategies may have augmented the variability. Second, the SSD-CI subjects were not tested to determine whether they have residual hearing in the implanted ear, and knowing that information may be helpful in understanding the role of that ear in contributing to outcomes tested here. Another issue is the subjective measure of tinnitus handicap; after implantation subjects may be biased by the desired effects of the surgery and perhaps feel strongly about the effects without large change. This issue is not easily resolvable with current measures of tinnitus handicap but are nonetheless important to consider. Finally, the auditory motion task was only tested in the Acoustic + Cl condition, hence no reference for Acoustic-only performance is available from these listeners. Future work in our lab is aimed at implementing both listening conditions.
5. Summary
This study showed findings from 15 adult participants (ages 48 ± 12 years) with single sided deafness, 9 of whom received a Cl (SSD-Cl). ln some cases, the Cl provided reduced tinnitus handicap reports. Audiological data from all 15 participants showed that when listening to sentences in quiet, performance was excellent in the Acoustic and Acoustic + Cl conditions. ln noise, performance was similar in Acoustic and Acoustic + Cl in 4/6 participants tested, and slightly worse in the Acoustic + Cl in 2/6 participants. Sound localization ability in the Acoustic + Cl condition showed reduction in RMS error with the addition of the Cl compared to the Acoustic- only condition. Preliminary results with a new perceptual task measuring auditory motion perception suggest worse performance than that of NH listeners. Future work will aim to increase the number of participants, the amount of experience post-implantation and additional controls in the Acoustic-only condition.
Acknowledgements
The authors are grateful to Heath Jones, Helena Seol, Brian Gartrell, Melanie Buhr-Lawler, Erin Nelson, Tanvi Thakkar, and Emily Burg for contributing to some of our data collection and analysis. We are also grateful to Drs. Samuel Gubbels, Douglas Sladen, Thomas Roland and Susan Waltzman for referring some of the early participants to us. This work was supported by the National lnstitutes of Deafness and Communicative Disorders [grant number R01 DC 003083 to RYL] and in by a core grant to the Waisman Center from the National lnstitute of Child Health and Human Development [U54 HD090256].
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016Zj.heares.2018.04.004.
References
- Arndt S, Aschendorff A, Laszig R, Beck R, Schild C, Kroeger S, Ihorst G, Wesarg T, 2011. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol. Neurotol. 32, 39–47. [DOI] [PubMed] [Google Scholar]
- Arts RA, George EL, Stokroos RJ, Vermeire K, 2012. Cochlear implants as a treatment of tinnitus in single-sided deafness. Curr. Opin. Otolaryngol. Head Neck Surg. 20, 398–403. [DOI] [PubMed] [Google Scholar]
- Baguley D, Bird J, Humphriss R, Prevost A, 2006. The evidence base for the application of contralateral bone anchored hearing aids in acquired unilateral sensorineural hearing loss in adults. Clin. Otolaryngol. 31, 6–14. [DOI] [PubMed] [Google Scholar]
- Balkany T, Hodges A, Telischi F, Hoffman R, Madell J, Parisier S, Gantz B, Tyler R, Peters R, Litovsky R, 2008. William house cochlear implant study group: position statement on bilateral cochlear implantation. Otology & neu- rotology: official publication of the american otological society. American Neurotology Society [and] European Academy of Otology and Neurotology 29, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein JG, Goupell MJ, Schuchman G.l., Rivera AL, Brungart DS, 2016. Having two ears facilitates the perceptual separation of concurrent talkers for bilateral and single-sided deaf cochlear implantees. Ear Hear. 37, 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauert J, 1997. Spatial Hearing: the Psychophysics of Human Sound Localization. Mit press. [Google Scholar]
- Buechner A, Brendel M, Lesinski-Schiedat A, Wenzel G, Frohne-Buechner C, Jaeger B, Lenarz T, 2010. Cochlear implantation in unilateral deaf subjects associated with ipsilateral tinnitus. Otol. Neurotol. 31,1381–1385. [DOI] [PubMed] [Google Scholar]
- Cabral F Junior, Pinna MH, Alves RD, Malerbi AF d. S, Bento RF, 2016. Cochlear implantation and single-sided deafness: a systematic review of the literature. Int. Arch. Otorhinolaryngol. 20, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson ML, Sladen DP, Gurgel RK, Tombers NM, Lohse CM, Driscoll CL, 2018. Survey of the american neurotology society on cochlear implantation: Part 1, candidacy assessment and expanding indications. Otol. Neurotol. 39, e12–e19. [DOI] [PubMed] [Google Scholar]
- Crew JD, Galvin III JJ, Landsberger DM, Fu QJ, 2015. Contributions of electric and acoustic hearing to bimodal speech and music perception. PLoS One 10, e0120279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon MT, Buss E, Anderson ML, King ER, Deres EJ, Buchman CA, Brown KD, Pillsbury HC, 2017. Cochlear implantation in cases of unilateral hearing loss: initial localization abilities. Ear Hear. 38, 611–619. [DOI] [PubMed] [Google Scholar]
- Döge J, Baumann U, Weissgerber T, Rader T, 2017. Single-sided deafness: impact of cochlear implantation on speech perception in complex noise and on auditory localization accuracy. Otol. Neurotol. 38, e563–e569. [DOI] [PubMed] [Google Scholar]
- Dorman MF, Loiselle LH, Cook SJ, Yost WA, Gifford RH, 2016. Sound source localization by normal-hearing listeners, hearing-impaired listeners and cochlear implant listeners. Aud. and Neurotol. 21, 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CC, Tyler RS, Witt SA, 2005. Benefit of wearing a hearing aid on the unimplanted ear in adult users of a cochlear implant. J. Speech Lang. Hear. Res. 48, 668–680. [DOI] [PubMed] [Google Scholar]
- Dunn CC, Tyler RS, Oakley S, Gantz BJ, Noble W, 2008. Comparison of speech recognition and localization performance in bilateral and unilateral cochlear implant users matched on duration of deafness and age at implantation. Ear Hear. 29, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JB, Shepherd RK, Nayagam DA, Wise AK, Heffer LF, Landry TG, Irvine DR, 2014. Effects of deafness and cochlear implant use on temporal response characteristics in cat primary auditory cortex. Hear. Res. 315,1–9. [DOI] [PubMed] [Google Scholar]
- Firszt JB, Reeder RM, Holden LK, Dwyer NY, Gotter B, Mispagel K, Potts L, Vanderhoof S, Holden T, Brenner C, 2018. Results in adult cochlear implant recipients with varied asymmetric hearing: a prospective longitudinal study of speech recognition, localization, and participant report. Ear Hear. 33, 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JR, Eggleston JL, Reavis KM, McMillan GP, Reiss LA, 2016. Effects of removing low-frequency electric information on speech perception with bimodal hearing. J. Speech Lang. Hear. Res. 59, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Galvin JJ, Wang X, 2017. Integration of acoustic and electric hearing is better in the same ear than across ears. Sci. Rep. 7, 12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartrell BC, Jones HG, Kan A, Buhr-Lawler M, Gubbels SP, Litovsky RY, 2014. Investigating long-term effects of cochlear implantation in single-sided deafness: a best practice model for longitudinal assessment of spatial hearing abilities and tinnitus handicap. Otology & neurotology: official publication of the American Otological Society. American Neurotology Society [and] European Academy of Otology and Neurotology 35,1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford RH, Grantham DW, Sheffield SW, Davis TJ, Dwyer R, Dorman MF, 2014. Localization and interaural time difference (ITD) thresholds for cochlear implant recipients with preserved acoustic hearing in the implanted ear. Hear. Res. 312, 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon K, Henkin Y, Kral A, 2015. Asymmetric hearing during development: the aural preference syndrome and treatment options. Pediatrics 136, 141–153. [DOI] [PubMed] [Google Scholar]
- Grantham DW, 1986. Detection and discrimination of simulated motion of auditory targets in the horizontal plane. J. Acoust. Soc. Am. 79, 1939–1949. [DOI] [PubMed] [Google Scholar]
- Grantham DW, Ashmead DH, Ricketts TA, Labadie RF, Haynes DS, 2007. Horizontal-plane localization of noise and speech signals by postlingually deafened adults fitted with bilateral cochlear implants. Ear Hear. 28, 524–541. [DOI] [PubMed] [Google Scholar]
- Grossmann W, Brill S, Moeltner A, Mlynski R, Hagen R, Radeloff A, 2016. Cochlear implantation improves spatial release from masking and restores localization abilities in single-sided deaf patients. Otol. Neurotol. 37, 658–664. [DOI] [PubMed] [Google Scholar]
- Grothe B, Pecka M, McAlpine D, 2010. Mechanisms of sound localization in mammals. Physiol. Rev. 90, 983–1012. [DOI] [PubMed] [Google Scholar]
- Guérit F, Santurette S, Chalupper J, Dau T, 2014. Investigating interaural frequency-place mismatches via bimodal vowel integration. Trends Hear 18, 1 —10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JD, Sergeant RL, 1971. Monaural/binaural minimum audible angles for a moving sound source. J. Speech Lang. Hear. Res. 14, 618–629. [DOI] [PubMed] [Google Scholar]
- Ihlefeld A, Carlyon RP, Kan A, Churchill TH, Litovsky RY, 2015. Limitations on monaural and binaural temporal processing in bilateral cochlear implant listeners. J. Assoc. Res. Otolaryngol 16, 641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaiah A, Vongpaisal T, King AJ, Hartley DE, 2014. Multisensory training im-proves auditory spatial processing following bilateral cochlear implantation. J. Neurosci. 34, 11119–11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H, Kan A, Litovsky RY, 2014. Comparing sound localization deficits in bilateral cochlear-implant users and vocoder simulations with normal-hearing listeners. Trends Hear 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris P, Yin TC, 2007. A matter of time: internal delays in binaural processing. Trends Neurosci. 30, 70–78. [DOI] [PubMed] [Google Scholar]
- Kan A, Litovsky RY, 2015. Binaural hearing with electrical stimulation. Hear. Res. 322, 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan A, Stoelb C, Litovsky RY, Goupell MJ, 2013. Effect of mismatched place-of- stimulation on binaural fusion and lateralization in bilateral cochlear-implant users. J. Acoust. Soc. Am. 134, 2923–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Johnstone PM, Godar SP, 2006. Benefits of bilateral cochlear im-plants and/or hearing aids in children. Int. J. Audiol. 45 (1), S78–S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Parkinson A, Arcaroli J, 2009. Spatial hearing and speech intelligibility in bilateral cochlear implant users. Ear Hear. 30, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Goupell MJ, Godar S, Grieco-Calub T, Jones GL, Garadat SN, Agrawal S, Kan A, Todd A, Hess C, Misurelli S, 2012. Studies on bilateral cochlear implants at the university of Wisconsin’s binaural hearing and speech laboratory. J. Am. Acad. Audiol. 23, 476–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CJ, Eddington DK, Colburn HS, Rabinowitz WM, 2003. Binaural sensi-tivity as a function of interaural electrode position with a bilateral cochlear implant user. J. Acoust. Soc. Am. 114,1565–1574. [DOI] [PubMed] [Google Scholar]
- Lovett RES, Vickers DA, Summerfield AQ, 2015. Bilateral cochlear implantation for hearing-impaired children: criterion of candidacy derived from an observational study. Ear Hear. 36, 14–23. [DOI] [PubMed] [Google Scholar]
- Macherey O, Carlyon RP, 2014. Cochlear implants. Curr. Biol. 24, R878–R884. [DOI] [PubMed] [Google Scholar]
- Mertens G, De Bodt M, Van de Heyning P, 2016. Cochlear implantation as a long-term treatment for ipsilateral incapacitating tinnitus in subjects with unilateral hearing loss up to 10 years. Hear. Res. 331,1–6. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Green DM, 1991. Sound localization by human listeners. Annu. Rev. Psychol. 42, 135–159. [DOI] [PubMed] [Google Scholar]
- Mok M, Grayden D, Dowell RC, Lawrence D, 2006. Speech perception for adults who use hearing aids in conjunction with cochlear implants in opposite ears. J. Speech Lang. Hear. Res. 49, 338–351. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health, 2016. Cochlear Implants (NIH Publication No. 00–4798). Rockville, MD.
- Neuman AC, Haravon A, Sislian N, Waltzman SB, 2007. Sound-direction identification with bilateral cochlear implants. Ear Hear. 28, 73–82. [DOI] [PubMed] [Google Scholar]
- Nopp P, Schleich P, D’haese P, 2004. Sound localization in bilateral users of MED- El COMBI 40/40+ cochlear implants. Ear Hear. 25, 205–214. [DOI] [PubMed] [Google Scholar]
- Perrott DR, Marlborough K, 1989. Minimum audible movement angle: marking the end points of the path traveled by a moving sound source. J. Acoust. Soc. Am. 85, 1773–1775. [DOI] [PubMed] [Google Scholar]
- Peters BR, Wyss J, Manrique M, 2010. Worldwide trends in bilateral cochlear implantation. Laryngoscope 120. [DOI] [PubMed] [Google Scholar]
- Poon BB, Eddington DK, Noel V, Colburn HS, 2009. Sensitivity to interaural time difference with bilateral cochlear implants: development over time and effect of interaural electrode spacing. J. Acoust. Soc. Am. 126, 806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkki V, 1997. Virtual sound source positioning using vector base amplitude panning. J. Audio Eng. Soc. 45, 456–466. [Google Scholar]
- Rahne T, Plontke SK, 2016. Functional result after cochlear implantation in children and adults with single-sided deafness. Otol. Neurotol. 37, e332–e340. [DOI] [PubMed] [Google Scholar]
- Ramsden JD, Papsin BC, Leung R, James A, Gordon KA, 2009. Bilateral simultaneous cochlear implantation in children: our first 50 cases. Laryngo-scope 119, 2444–2448. [DOI] [PubMed] [Google Scholar]
- Reiss LA, Eggleston JL, Walker EP, Oh Y, 2016. Two ears are not always better than one: mandatory vowel fusion across spectrally mismatched ears in hearing-impaired listeners. J. Assoc. Res. Otolaryngol 17, 341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Távora-Vieira D, De Ceulaer G, Govaerts PJ, Rajan GP, 2015. Cochlear implan-tation improves localization ability in patients with unilateral deafness. Ear Hear. 36, e93–e98. [DOI] [PubMed] [Google Scholar]
- Tillein J, Hubka P, Kral A, 2016. Monaural congenital deafness affects aural dominance and degrades binaural processing. Cerebr. Cortex 26,1762–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokita J, Dunn C, Hansen MR, 2014. Cochlear implantation and single sided deafness. Curr. Opin. Otolaryngol. Head Neck Surg. 22, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Heyning P, Vermeire K, Diebl M, Nopp P, Anderson I, De Ridder D, 2008. Incapacitating unilateral tinnitus in single-sided deafness treated by cochlear implantation. Ann. Otol. Rhinol. Laryngol. 117, 645–652. [DOI] [PubMed] [Google Scholar]
- Van de Heyning P, Távora-Vieira D, Mertens G, Van Rompaey V, Rajan GP, Müller J, Hempel JM, Leander D, Polterauer D, Marx M, 2016. Towards a unified testing framework for single-sided deafness studies: a consensus paper. Aud. and Neurotol. 21, 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoesel RJ, 2004. Exploring the benefits of bilateral cochlear implants. Aud. and Neurotol. 9, 234–246. [DOI] [PubMed] [Google Scholar]
- Vermeire K, Van de Heyning P, 2009. Binaural hearing after cochlear implantation in subjects with unilateral sensorineural deafness and tinnitus. Aud. and Neu- rotol. 14,163–171. [DOI] [PubMed] [Google Scholar]
- Vermeire K, Nobbe A, Schleich P, Nopp P, Voormolen MH, Van de Heyning PH, 2008. Neural tonotopy in cochlear implants: an evaluation in unilateral cochlear implant patients with unilateral deafness and tinnitus. Hear. Res. 245, 98–106. [DOI] [PubMed] [Google Scholar]
- Veugen LC, Hendrikse MM, van Wanrooij MM, Agterberg MJ, Chalupper J, Mens LH, Snik AF, van Opstal AJ, 2016. Horizontal sound localization in cochlear implant users with a contralateral hearing aid. Hear. Res. 336, 72–82. [DOI] [PubMed] [Google Scholar]
- Wess JM, Brungart DS, Bernstein JG, 2017. The effect of interaural mismatches on contralateral unmasking with single-sided vocoders. Ear Hear. 38, 374–386. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Dorman MF, Woldorff MG, Tucci DL, 2011. Cochlear implants: matching the prosthesis to the brain and facilitating desired plastic changes in brain function. Prog. Brain Res. 194, 117–129. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitler DM, Dorman MF, Natale SJ, Loiselle L, Yost WA, Gifford RH, 2015. Sound source localization and speech understanding in complex listening en-vironments by single-sided deaf listeners after cochlear implantation. Otology & neurotology: official publication of the American Otological Society. American Neurotology Society [and] European Academy of Otology and Neurotology 36, 1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon A, Smulders YE, Ramakers GG, Stegeman I, Smit AL, Zanten GA, Stokroos RJ, Hendrice N, Free RH, Maat B, 2016. Effect of unilateral and simultaneous bilateral cochlear implantation on tinnitus: a prospective study. Laryngoscope 126, 956–961. [DOI] [PubMed] [Google Scholar]


