Stem cells derived from adult tissues have long been considered multipotent, able to differentiate into a limited number of cell types found in their tissue of origin. Embryonic stem cells, in contrast, are pluripotent, which may differentiate into almost all cell types. With the ability to create induced pluripotent stem cells from somatic cells now available, the properties of multipotent stem cells are being re-evaluated. If adult cells may be reverted to pluripotent stem cells, can multipotent stem cells also be manipulated towards pluripotency? Advancements in biotechnology now allow for better methods to investigate stem cell plasticity, such as the relative influence of external versus intrinsic factors on cell fate. Recent studies indicate that adult neural stem cells (NSCs) demonstrate greater plasticity under certain conditions, resulting in the derivation of a variety of cell types including muscle, hematopoietic, and epithelial cells. This suggests that NSCs may provide a potential source of rare cell types for clinical application as an alternative to embryonic stem cells. Producing rare cell types from NSCs rather than embryonic stem cells avoids the ethical issues surrounding the use of this cell type. Further, NSCs may be an advantageous source compared to induced pluripotent stem cells, which are difficult to create, expensive, and time-consuming to develop.
Adult NSCs have the ability to form neurons, astrocytes, and oligodendrocytes in vitro. More recently, evidence has arisen which indicates adult NSCs may have extended plasticity. Studies have demonstrated differentiation into cell types of all three germ layers through a variety of methods.
Investigations of adult neural progenitor plasticity: There are a number of methods available to test for stem cell plasticity, as demonstrated in Figure 1. The chimera assay is the most open-ended approach. Adult stem cells are transplanted into a developing embryo at a pre-gastrulation stage. Growing in the midst of embryonic stem cells, the adult stem cells are exposed to a wide variety of signals in specific temporal patterns that may direct them down alternative fates. Development of the animal results in the formation of a chimera, an animal containing functioning cells from another organism (Figure 1A). The transplanted stem cells may be found within the tissue of their origin, or, if demonstrating expanded plasticity, be found in non-traditional tissues. In an in vitro version of this assay, adult cells are injected into a blastula, embryoid body, or are co-cultured with embryonic stem cells. This method, while simpler than performing experiments with live animals, does not allow for observation of the full developmental process due to the current limitations of in vitro organism development technology. However, it does permit close observation of cell behavior immediately following transplantation and direct measurement of fate-determining factors.
Figure 1.
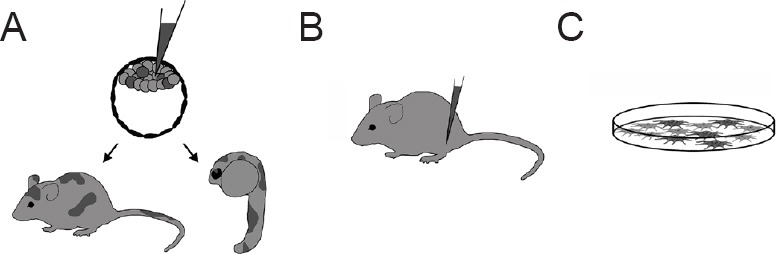
Methods for assessing adult stem cell plasticity.
(A) The chimera assay tests the full differentiation capacity of stem cells. The stem cells of interest (dark gray) are transplanted into the early developing embryo. Mice, chicks, swine, and now zebrafish have been utilized in this model. Plasticity is demonstrated when the transplanted cells are found with new phenotypes functioning outside of their tissue of origin. (B) Adult stem cells have been injected into specific tissues of adult mice to test for plasticity toward a particular fate. (C) Co-culture of adult stem cells with somatic cells or secreted factors also tests for differentiation toward a specific cell type.
Adult neural stem cell plasticity demonstrated by the chimera assay was first exhibited in 2000. Adult mouse neural progenitors were transplanted into mouse blastocysts, generating chimeric animals. Characterization by immunohistochemistry demonstrated differentiation into cardiac muscle cells, hepatocytes, and epithelial cells. The neural progenitors were also injected into chick embryos, a process called xenotransplantation (xeno referring to cross-species). Adult neural progenitor-derived cells were observed, forming chimeric ectodermal, endodermal, and mesodermal tissues (Clarke et al., 2000). A following study published in 2004 found contradicting results. Transplantation of fetal mouse neural progenitor cells into mouse blastocysts did not result in chimeric animals. Further investigation following blastula development in vitro determined that the progenitors rapidly differentiated into glial cells, preventing assessment of plasticity (Gréco et al., 2004). Fetal porcine neural progenitor cells transplanted into 4- and 9-cell stage embryos of the same species did not form chimeras, as well (Zhao et al., 2012). Tests for adult neural stem cell plasticity using the chimera assay have been performed using embryonic zebrafish, as well.
Xenotransplantation of mammalian cells into zebrafish has recently been developed as a rapid method for the study of cell behavior and fate. The fate of transplanted cells may be observed in real-time as zebrafish are transparent at early stages. Ex-utero development avoids the need for embryo implantation into surrogate mothers, further aiding observation. The immature zebrafish immune system at this stage also prevents donor cell rejection. Similar to the results in mice, findings of plasticity following xenotransplantation into zebrafish are variable.
Fetal mouse neural progenitor cells transplanted into blastula-stage zebrafish by Xiao et al. (2016) were later found in multiple locations, including mesodermally-derived tissues such as heart and blood, epithelial, and endodermal tissues. Although no immunohistochemical characterization of these cells was performed, cells in the epidermis did display an epithelial morphology. Subsequent co-culture of the neural progenitor cells with mouse skin cells resulted in the formation of keratin1-positive cells (Xiao et al., 2016).
A recent study published by Sandquist and colleagues in 2018 also demonstrated chimerism following transplantation of adult rat neural progenitors into embryonic zebrafish. The majority of cells found outside the CNS were located in the epidermis, with cells also observed in skeletal, cardiac muscle and facial cartilage. However, immunohistochemical analysis indicated that the majority of transplanted cells retained their neural phenotypes despite their locations outside the central nervous system, with positive immunolabeling for class III β-tubulin (Sandquist et al., 2018). While investigating adult neural stem cell plasticity by the chimera assay has resulted in conflicting findings, attempts to direct these cells down specific non-neural lineages have been met with more success.
One in vivo approach to test the ability of adult stem cells to acquire a specific fate is to inject stem cells into the tissue of the type desired of a living animal (Figure 1B). This allows for stem cells to be exposed to a set of factors likely to induce differentiation without requiring previous identification. The limitation to this method is that only a few alternative fates may be tested at a time.
The first hint that adult NSCs could demonstrate expanded cell fate was published in 1999, when adult and fetal neural progenitors were intravenously injected into mice. The cells recapitulated the hematopoietic system, with positive immunolabeling for T lymphocyte, B lymphocyte, and myeloid cell markers (Bjornson et al., 1999). However, a following study was unable to replicate these findings despite injection of fetal neural progenitors into 128 animals (Morshead et al., 2002). In another study, neural progenitor differentiation into skeletal muscle cells has been demonstrated after transplantation into regenerating muscle of adult mice (Galli et al., 2000).
The most direct method to test plasticity is to attempt the guided differentiation of adult stem cells toward a specific alternative fate. This is usually performed in vitro by co-culture with mature cells of the type which the stem cell is being guided toward (Figure 1C). Culture of adult stem cells with a variety of factors secreted by the cell type of interest has also been performed. These methods allow for controlled administration of factors which are known to promote differentiation of a particular fate.
Co-culture of neural progenitors at low numbers with mature cell types has prompted differentiation into epithelial cells as previously mentioned, as well as vascular endothelial and muscle cells (Bani-Yaghoub et al., 2004; Wurmser et al., 2004; Hori et al., 2005). Adult neural progenitors have been induced to differentiate into other cell types with exposure to secreted factors. Insulin exposure caused fetal neural progenitors to exhibit markers of muscle cells and to begin spontaneously contracting in culture in a study by Bani-Yaghoub and colleagues. These more direct approaches, while limited to the study of individual fates, appear to support findings from chimera studies demonstrating differentiation of adult NSCs down alternative fates.
Discussion: It appears that while plasticity is possible, the extent to which it can occur is unknown. In vivo studies using the chimera assay, which can demonstrate significant levels of plasticity, have been variable. The ability for adult NSCs to differentiate into a limited number of mature cell types in vitro, however, is likely.
Contradicting results may be due to cell fusion events. Neural progenitors can fuse with embryonic stem cells, creating cells with large nuclei containing multiple nucleoli. Few of the published studies on adult NSC plasticity have tested for the possibility of cell fusion. While the occurrence of cell fusion events is likely low, it is sufficient to say that the extent of adult stem cell plasticity may be overestimated. Future investigations can detect cell fusion events by using adult NSCs derived from the brains of female animals and male recipients. Cells exhibiting plasticity and lacking the Y chromosome can then be confirmed as deriving from adult neural stem cells and not cell fusion (Gréco and Recht, 2003).
Variable findings may also be due to incompatibility in differentiation paradigms between adult and fetal cells. The transplanted cells may not be exposed to the correct combination of differentiation factors over the proper time frame. Xenotransplantation further complicates this issue, as the length of development can vary greatly between the animals from which the NSCs were isolated and the animals into which they were transplanted, as is in the case of rodent and zebrafish. In addition, the optimal temperature for zebrafish growth is significantly different than that of mammalian cells.
Behavior of adult NSCs may be varied due to the location in the brain from which they were derived, the age of the animals from which they were isolated, and the methods of culture prior to transplantation. A variety of NSC populations have been used, including cells isolated from the subventricular zone and ependymal layers of the lateral ventricle and the subgranular zone of the hippocampus. Stem cells from these locations may exhibit differing levels of plasticity. Further, many studies used progenitor cells isolated from whole or partial brain dissociation protocols. This can result in heterogeneous cell populations containing both neural stem and progenitor cells with differing potential. It may be that only a subset of the stem cell population is able to exhibit plasticity. Better characterization and stringent isolation protocols for adult NSCs prior to transplantation may reveal the reasons for the low levels of chimerism and high variability observed.
Neural stem cell plasticity has been investigated using progenitor cells from both fetal and adult animals, with no clear evidence of its impact as of yet. Adult NSC behavior is also dependent upon its culture as neurospheres or a monolayer, with published studies using both methods. In addition, differing results have been observed with cells transplanted as whole or dissociated neurospheres. Future investigations controlling for these variables may provide more consistent results.
In a review by Gréco and Recht (2003), on the topic of neural stem cell plasticity, it is recommended that future studies use the following three parameters to define plasticity: incorporation, differentiation, and functionality. Many investigations have demonstrated chimerism by incorporation, in which the transplanted neural progenitors are found outside the central nervous system. The majority perform some type of characterization to determine if the progenitors indeed differentiated into alternative cell types, though this could be improved in regard to testing for cell fusion events. Very few have demonstrated that the neural progenitors differentiated into mature cell types which contribute to the function of their surrounding tissue. When these three considerations have been addressed, the use of stem cell plasticity for therapeutic applications may be considered, such as generation of rare cell types for transplantation.
Adult neural stem cells exhibit greater differentiation potential than originally thought due to advancements in techniques to test plasticity. However, the full extent of this plasticity is yet to be determined. Future studies which take into consideration the potential for stem cell fusion and carefully characterize the neural stem cell population and its resulting fate are likely to resolve this issue. At that point, optimization of differentiation protocols may then be performed for therapeutic application of stem cell transplantation.
The authors would like to thank Dr. Jeffrey J. Essner (Genetics, Development and Cell Biology, Iowa State University, Ames, IA, USA) for his intellectual contributions to this article.
Additional file: Open peer review report 1 (145.6KB, pdf) .
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Kyle D. Fink, University of California Davis Health System, USA.
P-Reviewer: Fink KD; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Bani-Yaghoub M, Kendall SE, Moore DP, Bellum S, Cowling RA, Nikopoulos GN, Kubu CJ, Vary C, Verdi JM. Insulin acts as a myogenic differentiation signal for neural stem cells with multilineage differentiation potential. Development. 2004;131:4287–4298. doi: 10.1242/dev.01295. [DOI] [PubMed] [Google Scholar]
- 2.Bjornson CRR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- 3.Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlström J, Lendahl U, Frisén J. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- 4.Galli R, Borello U, Gritti A, Minasi MG, Bjornson C, Coletta G, Vescovi AL. Skeletal myogenic potential of human and mouse neural stem cells. Nat Neurosci. 2000;3:986–991. doi: 10.1038/79924. [DOI] [PubMed] [Google Scholar]
- 5.Gréco B, Recht L. Somatic plasticity of neural stem cells: fact or fancy? J Cell Biochem. 2003;88:51–56. doi: 10.1002/jcb.10314. [DOI] [PubMed] [Google Scholar]
- 6.Gréco B, Low HP, Johnson EC, Salmonsen RA, Gallant J, Jones SN, Ross AH, Recht LD. Differentiation prevents assessment of neural stem cell pluripotency after blastocyst injection. Stem Cells. 2004;22:600–608. doi: 10.1634/stemcells.22-4-600. [DOI] [PubMed] [Google Scholar]
- 7.Hori Y, Gu X, Xie X, Kim SK. Differentiation of insulin-producing cells from human neural progenitor cells. PLoS Med. 2005;2:e103. doi: 10.1371/journal.pmed.0020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morshead CM, Benveniste P, Iscove NN, van der Kooy D. Hematopoietic competence is a rare property of neural stem cells that may depend on genetic and epigenetic alterations. Nat Med. 2002;8:268–273. doi: 10.1038/nm0302-268. [DOI] [PubMed] [Google Scholar]
- 9.Sandquist EJ, Essner JJ, Sakaguchi DS. Xenotransplantation of adult hippocampal neural progenitors into the developing zebrafish for assessment of stem cell plasticity. PLoS One. 2018;13:e0198025. doi: 10.1371/journal.pone.0198025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wurmser AE, Nakashima K, Summers RG, Toni N, D’Amour KA, Lie DC, Gage FH. Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature. 2004;430:350–356. doi: 10.1038/nature02604. [DOI] [PubMed] [Google Scholar]
- 11.Xiao C, Qian M, Yin C, Zhang Y, Hu H, Yao S. A zebrafish mosaic assay to study mammalian stem cells in real time in vivo. J Mol Histol. 2016;47:437–444. doi: 10.1007/s10735-016-9688-x. [DOI] [PubMed] [Google Scholar]
- 12.Zhao MT, Yang X, Lee K, Mao J, Teson JM, Whitworth KM, Samuel MS, Spate LD, Murphy CN, Prather RS. The in vivo developmental potential of porcine skin-derived progenitors and neural stem cells. Stem Cells Dev. 2012;21:2682–2688. doi: 10.1089/scd.2012.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


