Table 1. Optimization of reaction conditions a .
| ||
| Entry | Variations from the standard conditions | Yield b |
| 1 | None | 83% |
| 2 | w/o HE | 0% c |
| 3 | Dark | 0% |
| 4 | Dark, 80 °C | 0% c |
| 5 | w/o DABCO | 22% c |
| 6 | TMEDA, instead of DABCO | 43% |
| 7 | DBU, instead of DABCO | 19% |
| 8 | Et3N, instead of DABCO | 26% |
| 9 | Pyridine, instead of DABCO | 19% |
| 10 | Cs2CO3, instead of DABCO | 21% |
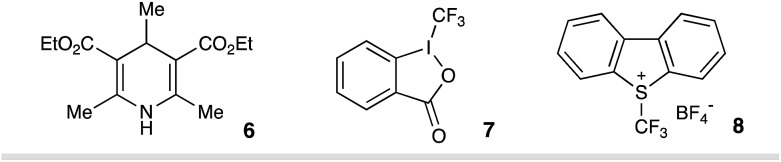
| 11 | 6, instead of HE | 25% |
| 12 | 7, instead of 4 | 19% |
| 13 | 8, instead of 4 | 25% |
| ||
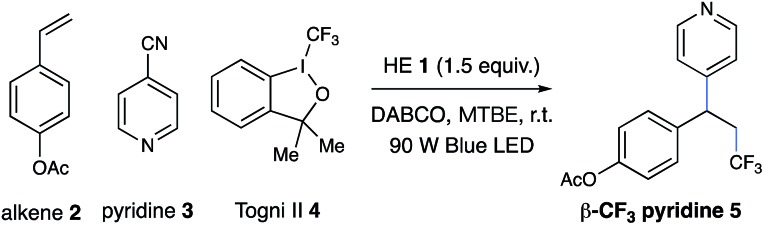
aReaction conditions: styrene 2 (0.1 mmol), 4-cyanopyridine 3 (2.0 equiv.), Togni reagent 4 (1.5 equiv.), Hantzsch ester (HE, 1.5 equiv.), DABCO (1.5 equiv.), MTBE [0.05 M], 90 W blue LED, and rt.
bYields were determined by 19F NMR using an internal standard.
cMajor byproducts determined were dimers of benzylic radicals; see the ESI for details. DABCO: 1,4-diazabicyclo[2.2.2]octane; MTBE: methyl tert-butyl ether.
