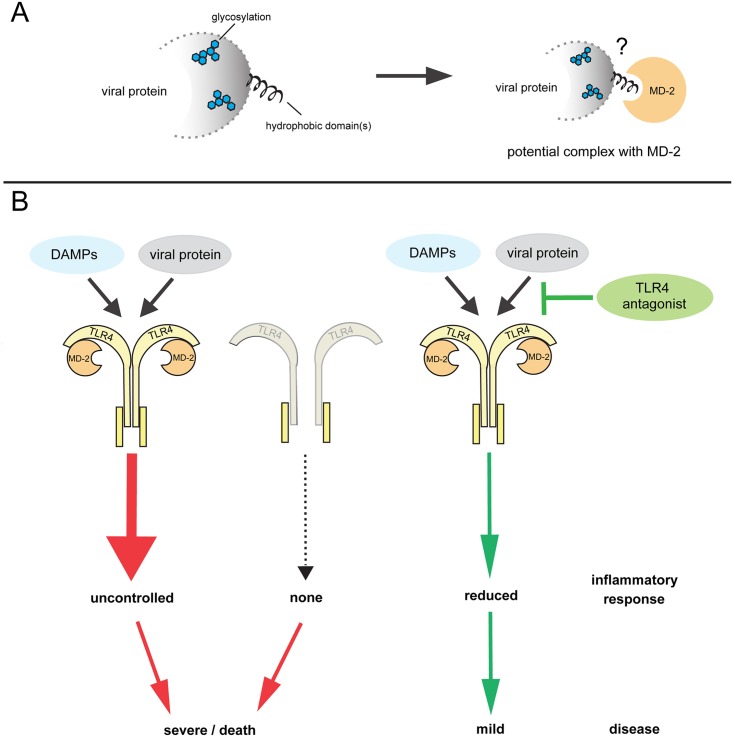
Fig 1. TLR4 in viral pathogenesis.
(A) Viral proteins known to activate TLR4 are membrane-bound or membrane-associated, contain hydrophobic domains, and are glycosylated. Although it remains to be determined how these viral proteins interact with the MD-2/TLR4 complex, data from known TLR4 activators suggest that the hydrophobic domains of these viral proteins might bind in the hydrophobic pocket of MD-2. Glycans on the viral protein could be involved in stabilizing the MD-2/TLR4 complex to enhance TLR4 signaling. Compared to the well-described interaction of TLR4 complexes with relatively small bacterial LPSs, the mechanism by which these complexes recognize large viral glycoproteins to trigger downstream signaling remains largely unexplored. (B) Viral proteins and host DAMPs, which accumulate in response to cellular stress during viral infection, have been linked to TLR4 activation during virus infection. Both uncontrolled activation of TLR4 and TLR4 knockout are associated with severe disease, whereas reducing the TLR4-mediated inflammatory response using TLR4 inhibitors mitigates disease symptoms, offering potential treatment options for various severe viral infections. DAMP, damage-associated molecular pattern; LPS, lipopolysaccharide; MD-2, myeloid differentiation factor 2; TLR4, toll-like receptor 4.