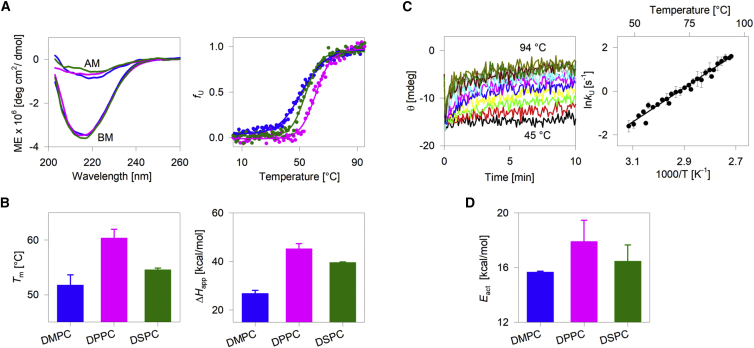
Figure 1.
Effect of lipid-diacyl-chain length on hVDAC2 stability. (A, left) Representative far-UV CD wavelength scans of folded hVDAC2 at 4°C. BM, before thermal denaturation; AM, after thermal denaturation. (A, right) Dependence of the unfolded protein fraction (fU) on temperature, derived from far-UV CD thermal unfolding at 215 nm. Fits of the data from DMPC (blue), DPPC (pink), and DSPC (green) bicelles to a two-state thermal denaturation model are in solid lines. (B) Comparison of the Tm and ΔHapp derived from the thermal unfolding measurements reveals that stability is highest in DPPC. Error bars represent the SD calculated from three independent experiments. The significance of the differences was measured for DMPC and DSPC with respect to DPPC using t-test. In the case of Tm, p-values are 0.0005 for DMPC-DPPC and 0.0012 for DSPC-DPPC. For ΔHapp, p-values are 0.0002 for DMPC-DPPC and 0.01 for DSPC-DPPC. (C, left) Representative isotherms for the unfolding kinetics of hVDAC2 monitored in DMPC using far-UV CD (215 nm) at various temperatures from 46 to 94°C at 2°C intervals. The fit of each isotherm to a single exponential function provided the unfolding rate (kU) at that temperature. (C, right) A representative Arrhenius plot (in DMPC bicelles) obtained by plotting the ln kU against temperature. Fit (solid line) of the data to the Arrhenius equation yielded the activation energy (Eact). (D) Dependence of Eact of hVDAC2 on the acyl-chain length. Error bars represent the SD calculated from two independent experiments, with each experiment containing ∼25 independently measured rates. Overall, the hVDAC2 stability from Tm, ΔHapp, and Eact is highest in DPPC. The complete data are presented in Figs. S3 and S4. To see this figure in color, go online.