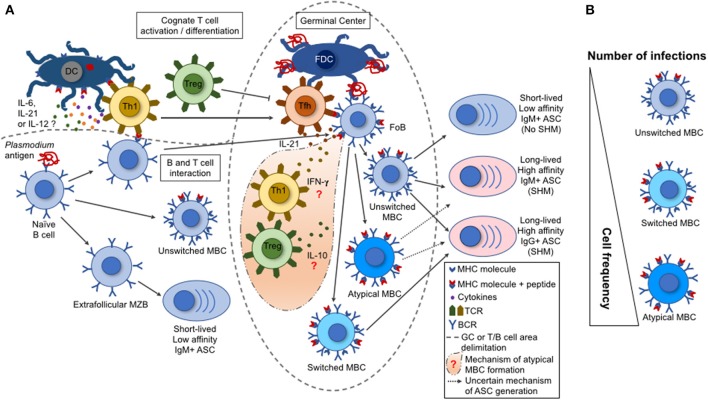
Figure 1.
B cell response triggered by malaria infection. (A) During a malaria infection, the naive B cells are activated by a Plasmodium antigen through the interaction with B cell receptors (BCR), leading to their differentiation into marginal zone B cells (MZB), follicular B cells (FoB), or unswitched memory B cell (MBCs). The switched and atypical MBCs are derived from the activation of FoBs within the germinal centers (GCs). Either the MZBs, or the unswitched, switched, or atypical MBCs can differentiate into antibody-secreting cells (ASCs). These ASCs range from short-lived, low-affinity, IgM+ to long-lived, high-affinity, IgM+ or IgG+. This variation depends on the type of interaction between a particular B cell with a T cell subset. The activated Th1 T cells migrate to the GCs, becoming follicular T helper cells (TFh) that help the GC reactions (acquisition of somatic hypermutations in V(D)J Ig genes and class switch by activated FoBs). Contrarily, the regulatory T cells (Tregs) have the potential to inhibit TFh cell differentiation and GC reactions. (B) A single parasite infection can induce the differentiation of multiple Plasmodium-specific B cell clones. However, the repeated parasite exposures shift the MBC frequencies with an increase for an atypical MBC over the unswitched or switched MBCs. This shift in cell frequency may interfere on the function of the secreted antibodies and, consequently, on the development of protective immunity.