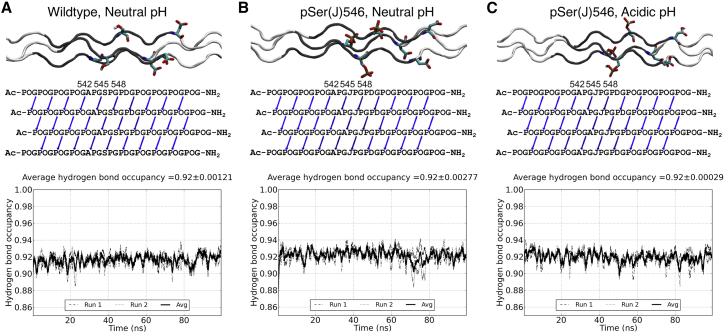
Figure 2.
(A) Molecular dynamics (MD) simulation results of the α1(I)3 wild-type (WT) Ser546 peptide at neutral pH, (B) α1(I)3 pSer546 at neutral pH, and (C) α1(I)3 pSer546 at acidic pH. Top: A side view of the triple helices in the end of the simulations is shown. The GPO triplets are colored white, and human sequence triplets are colored gray; Ser546/pSer546 and Asp are drawn in sticks. Middle: A diagram of the average occupancy of interchain NH···CO hydrogen bonds within the peptide is shown. In the sequences, phosphorylated serines are designated as J. Arrows are used to indicate hydrogen bonds, defined as a donor-acceptor distance <3.5 Å and a hydrogen-donor-acceptor angle <30° and based on the average of two 100 ns MD runs. Bottom: Occupancy of the interchain NH···CO hydrogen bonds in the human sequence region as a function of simulation time is shown. Hydrogen bond occupancies as a function of time during each of the two MD runs are depicted as dashed and dotted, respectively; the average of the two runs is shown in a solid line. A running average with a sliding window of 1 ns is used. To see this figure in color, go online.