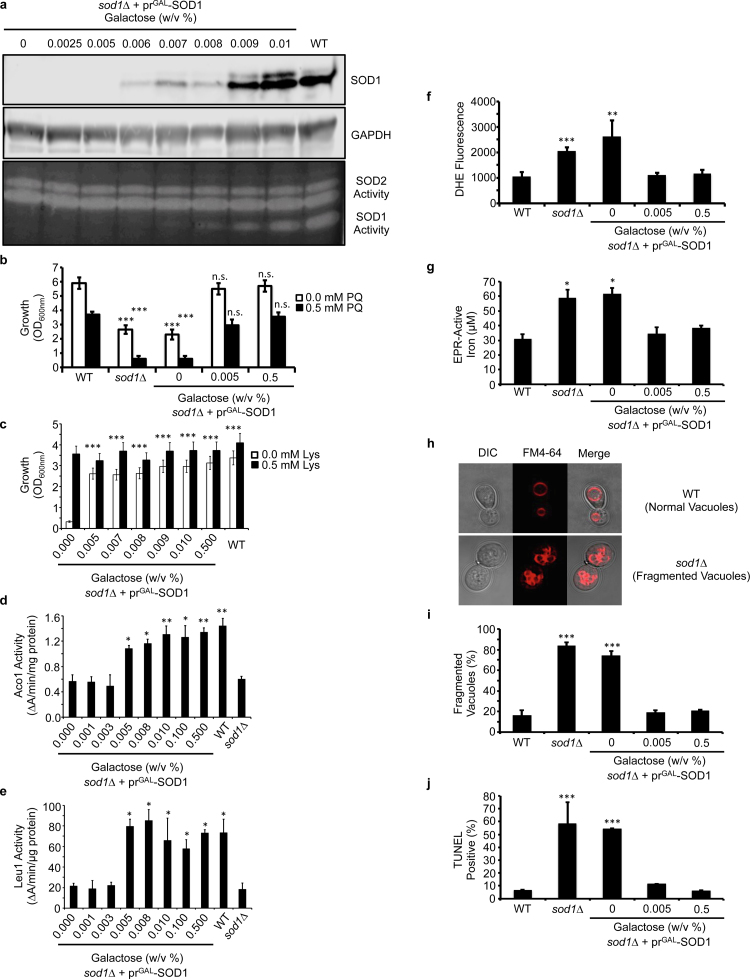
Fig. 1.
The vast majority of Sod1 is dispensable for protection against superoxide toxicity. (a) Titration of galactose (GAL) into cultures of sod1::LEU2 (sod1∆) cells expressing the GAL1 driven Sod1 expression vector (prGAL-SOD1; pAR1026) results in the expression of low (≤ .005% GAL), intermediate (0.006–0.008% GAL), and high (≥ 0.009% GAL) expression and activity of Sod1. The immunoblots and activity gels depicted are representative of multiple trials across different batches of media. (b) Paraquat (PQ) sensitivity of WT and sod1∆ cells compared to sod1∆ + prGAL-SOD1 cells expressing none (0% GAL), low (0.005% GAL) or high (0.5% GAL) levels of Sod1 as measured by solution turbidity. (c) Lysine (Lys) auxotrophy, (d) aconitase (Aco1) activity, and (e) isopropylmalate isomerase (Leu1) activity of sod1∆ + prGAL-SOD1 cells is measured as a function of Sod1 expression and compared to WT and/or sod1∆ cells. (f) DHE detectable superoxide is monitored in WT, sod1∆, and sod1∆ + prGAL-SOD1 cells expressing none (0% GAL), low (0.005% GAL), or high (0.5% GAL) Sod1. (g) EPR detectable labile Fe, (h and i) FM4–64 visualized vacuolar fragmentation, and (j) DNA damage using the TUNEL assay was monitored in WT, sod1∆, or sod1∆ + prGAL-SOD1 cells expressing none (0% GAL), low (0.005% GAL), or high (0.5% GAL) Sod1. In panels i and j, approximately ~100 cells were counted from each culture condition in triplicate and scored for (i) having single or multiple fragmented vacuoles, as depicted in panel h, or (j) being non-fluorescent or fluorescent in the FITC channel. Error bars indicate the average ± s.d. of triplicate (b, c, f, i, j) or duplicate (d, e, g) independent cultures. The statistical significance relative to WT (b, f, g, i, j) or sod1∆ + prGAL-SOD1 cells cultured with 0% GAL (c, d, e) is indicated by asterisks using an ordinary one-way ANOVA with Dunnett's post-hoc test. * P < 0.05, ** P < 0.01, *** P < 0.0001, n.s. = not significant.