Abstract
Research question
Is formulated and lyophilized, recombinant human Müllerian inhibiting substance, also known as anti-Müllerian hormone (AMH), suitable for the preparation of a WHO international standard to calibrate AMH immunoassays?
Design
The AMH content of a trial preparation, coded SS-581, was determined by five laboratories using seven immunoassay methods. Participants were requested to report the content of the preparation in terms of their method calibrators through the measurement of a minimum of five concentrations in the linear part of the dose-response curve. Participants were also asked to measure, concomitantly, a panel of six serum samples containing AMH at concentrations of 0.1–13.0 ng/ml.
Results
Across all assays, including two automated assays in development, the geometric mean content was 361.76 ng/ampoule with a geometric coefficient of variation (GCV%) of 39.95%. When measured by immunoassays that were commercially available at the time of the study, the mean content was 423.08 ng/ampoule, with a GCV% of 26.67%. The inter-method geometric means of five serum samples with an AMH concentration >0.3 ng/ml and measured concomitantly with dilutions of SS-581 varied with a range of GCV% of 14.90–22.35%, which may reflect the use of serum sample value transfer to calibrate current immunoassays, some of which use non-human AMH calibrators. The AMH in trial preparation SS-581 was shown to be biologically active in the Müllerian duct regression assay.
Conclusions
A reference material prepared using human recombinant AMH is a promising candidate for the preparation of an international standard for AMH for immunoassays calibrated to recombinant human AMH.
Keywords: Anti-Müllerian hormone, Immunoassay, International standard, Müllerian inhibiting substance, WHO, World Health Organization
Key message.
Establishment of an international standard to calibrate AMH immunoassays will serve to harmonize assays and support method development. A promising source material is recombinant AMH, which, when lyophilized is stable and retained immunoreactivity in all methods tested. Introduction of a standard requires consideration of developments in AMH measurement.
Alt-text: Unlabelled box
Introduction
Müllerian inhibiting substance (MIS), also known as anti-Müllerian hormone (AMH), is a homodimeric glycoprotein expressed by the Sertoli cells of the testes and the granulosa cells of early antral and antral ovarian follicles (Josso et al., 1993, Takahashi et al., 1986, Vigier et al., 1984). In females, serum concentrations of AMH are accepted as a biomarker of the dynamic reserve of growing follicles, the ovarian reserve, which is considered to be in equilibrium with the reserve of primordial follicles established early in life (Monniaux et al., 2014). Thus, AMH determinations are used to assess infertility and, as part of assisted reproductive therapies, support the use of tailored stimulation protocols to minimize ovarian hyperstimulation or optimize oocyte recovery. This has extended to the proposed use of a specific AMH immunoassay as a companion diagnostic to inform the individualized dosing of a human recombinant FSH therapeutic (Nyboe Andersen et al., 2017).
AMH is synthesized as a disulphide-linked homodimer. Each AMH monomer consists of 560 amino acids including a signal peptide (amino acid residues 1–24 of NCBI RefSeq NP_000470.2). Removal of the signal peptide generates a proprotein that is biologically inactive until proteolysis occurs between residues 451 and 452. Cleavage generates a N-terminal dimer (AMHN or AMH-Pro) and a C-terminal dimer (AMHC or AMH-Mature) which remain non-covalently associated (AMHN,C or AMH-Pro-Mature). Both cleaved and uncleaved forms have been detected in circulation, which has led to the suggestion that either cleavage is regulated during expression and secretion by gonadal tissue or that it occurs at the site of action. Alternatively, uncleaved AMH may have an as yet unidentified function (Pankhurst and McLennan, 2013, Pankhurst et al., 2016). Uncleaved recombinant AMH does not bind the extracellular fragment of the AMH receptor II (AMHRII) in vitro, as shown by Pierre et al. (2016). However, in the same study, it was also suggested that cleaved AMH, immunoaffinity-purified from serum, was also not receptor binding competent in vitro. These studies illustrate that the exact nature of AMH bioactivity remains unclear. However, it is thought that all AMH immunoassays in current clinical use detect total AMH (cleaved and uncleaved) in serum and as such, it is reported measures of total AMH that contribute to a clinical decision or diagnosis.
Immunoassays to detect AMH in patient serum have developed from early research assays to commercially available manual immunoassays and more recently, AMH assays on automated platforms. The development of AMH immunoassays, summarized by Dewailly et al. (2014), resulted in two manual immunoassays becoming available for widespread clinical use. These were from Immunotech-Coulter (IOT), which was calibrated to a standard curve of recombinant AMH assigned in pmol/l (Long et al., 2000) and from Diagnostic Systems Laboratories, which reported in ng/ml (Groome et al., 2011). The assays used different pairs of monoclonal antibodies raised to recombinant human AMH and different AMH calibrators. Reported serum AMH concentrations were method-specific, with the DSL assay providing values that were three- to four-fold lower than those determined by the IOT immunoassay (Bersinger et al., 2007, Freour et al., 2007).
On acquiring both assays, Beckman Coulter developed the AMH Gen II manual immunoassay by using the monoclonal antibodies, F2B 7/A and F2B 12/H, from the DSL ELISA but calibrating to the higher reporting values of the IOT immunoassay (Kumar et al., 2010). This was achieved by measuring 120 serum samples in the IOT immunoassay and using the concentrations obtained from the IOT standard curve of human recombinant AMH (Long et al., 2000) to calibrate the AMH Gen II assay and to assign values to the AMH Gen II calibrators, which are of bovine origin. In addition, the AMH Gen II assay underwent a protocol modification in 2013 (MHRA, 2013) to eliminate interference due to the binding of the complement component C3b to the capture antibody, F2B 12/H, which produced falsely low AMH values when fresh or freshly frozen sera were measured.
Independently of the Beckman Coulter immunoassay, Gopal and Kumar developed AMH immunoassays (Ansh Ultra-Sensitive AMH/MIS ELISA and picoAMH ELISA) using a pair of monoclonal antibodies which recognized linear epitopes in the AMHN and AMHC regions with a high affinity and specificity for AMH (Gopal and Ajay, 2014, Visser et al., 2013). To calibrate the assay, the concentration of a purified preparation of AMHC was determined by optical density at 280 nm. The purified and quantified AMHC region was then used to value assign the recombinant human AMH calibrators, although the method by which this was achieved is not disclosed. A recent study by Nelson et al. (2015) comparing the Ansh Ultra-Sensitive AMH/MIS ELISA to the modified AMH Gen II assay concluded that the AMH concentrations obtained for 83 archived serum samples were broadly comparable.
The first automated immunoassay for AMH was launched in 2014 by Roche for the Elecsys/cobas e platform and was based on monoclonal antibodies F2B 12/H and F2B 7/A (Gassner and Jung, 2014). Sample value transfer using 81 serum samples measured by the unmodified AMH Gen II ELISA protocol was used to assign AMH concentrations to the Elecsys/cobas e signal. A similar approach was used by Beckman Coulter to calibrate an automated immunoassay, also based on monoclonal antibodies F2B 12/H and F2B 7/A, for the Access platform; in this case, the modified version of the AMH Gen II assay was used to measure 239 serum samples (Demirdjian et al., 2016).
From these historical calibration exercises, the accepted estimation of a ‘nanogram of AMH’ for at least three commercially available assays appears to have originated from the IOT AMH ELISA standards. These comprised a ‘preparation of purified recombinant human AMH which was used to construct a standard curve ranging from 0.7 – 175 pmol/l’ (Long et al., 2000). The method by which the molar concentration of the calibrators was determined is not disclosed. Furthermore, for current immunoassays, the convention is to report AMH measurements in ng/ml using the widely cited conversion of 1 ng/ml = 7.14 pM. This conversion is derived from the apparent molecular weight of the glycosylated AMH dimer (140 kDa) as determined by non-reducing SDS-PAGE (Budzik et al., 1983, Cate et al., 1986, Vigier et al., 1984), which is considerably greater than the molecular mass of the AMH dimer determined from the peptide sequence (113 kDa). Yet the mass contributed by glycosylation of either native or recombinant AMH has not been accurately determined.
Despite the same antibodies being used in three of four commercially available assays, comparisons of these have indicated some discordance (Li et al., 2016, Nelson et al., 2015, Van Helden and Weiskirchen, 2015). A comparative study using 1729 routine samples showed that analyte recovery by the Elecsys AMH assay was 24–28% higher than by the modified AMH Gen II ELISA (Anckaert et al., 2016). These and previously reported between-method discrepancies have resulted in regular requests for the development of an international standard for AMH. To this end, a proposal from the National Institute for Biological Standards and Control (NIBSC, UK) was endorsed by the World Health Organization (WHO, 2015).
Development of a WHO international standard for AMH presents a number of challenges. First, it requires a source of purified AMH to prepare a homogenous batch of 2000–3000 ampoules containing a sufficient quantity of AMH to ensure consistent recovery by users. Secondly, it requires the identification of a formulation and lyophilization process which retains immunoreactivity, meets the long-term stability requirements of a WHO international standard and is commutable with representative patient samples. Thirdly, it requires a strategy to define the content of AMH in the ampoules. To further investigate these challenges, this paper describes the preparation and evaluation of a trial batch of ampoules containing formulated recombinant human AMH using a portion of a preparation of AMH donated to NIBSC for the purpose of preparing an international standard. The AMH content in the trial batch was determined in terms of method calibrators using marketed immunoassays or immunoassays in development.
Materials and methods
Bulk material and preparation of ampoules of a trial formulation coded SS-581
Purified recombinant human AMH was donated to NIBSC by Patricia Donahoe (Director) and Dr David Pépin of the Pediatric Surgical Research Laboratories, Massachusetts General Hospital, USA. As previously described (Pépin et al., 2013), AMH was expressed in CHO-K1 cells from a clone coded LR-MIS LR11 in which the cleavage recognition site had been mutagenized from RAQR/S to RARR/S (position 450 of NCBI RefSeq NP_000470.2) to increase the proportion of cleaved, bioactive AMH and the leader sequence substituted for the leader sequence of human serum albumin to increase the yield of expressed protein. Expressed protein was purified by immunoaffinity chromatography.
An aliquot of purified recombinant human AMH was measured by a commercially available manual AMH ELISA and used to prepare a 50 ml volume of a solution containing 0.24% (w/v) bovine casein, 0.50% (w/v) trehalose and nominally 1 μg/ml AMH. Siliconized 3 ml ampoules were filled with a 0.5 ml volume of formulation and the contents lyophilized using a 4-day cycle in a Virtis Genesis 25EL freeze dryer (Biopharma Ltd, Winchester, UK). Ampoules were stoppered under nitrogen and sealed. Ampoules, coded SS-581, were stored at –20°C.
Characterization of lyophilized product in SS-581
Ampoules of SS-581 were characterized by determination of the fill weight, dry weight, residual moisture by coulometric Karl Fischer titration (Mitsubishi CA100 coulometer, A1-Envirosciences Ltd, Blyth, UK) and headspace oxygen using frequency-modulated spectroscopy with a non-destructive laser headspace analyser (Lighthouse Instruments, Charlottesville, USA). The AMH content of the ampoules was estimated by a manual AMH ELISA and compared with a sample removed prior to lyophilization and stored at –70°C.
Assessment of the bioactivity of AMH in SS-581
Bioactivity of the lyophilized AMH in ampoules coded SS-581 was assessed by the graded organ culture bioassay for Müllerian inhibiting substance (MIS), as described in Pépin et al. (2013). This assay is uniquely specific to AMH and is considered the gold standard for the determination of AMH bioactivity (Price et al., 1979). Briefly, the material was reconstituted at 5 μg/ml and 2 μg/ml in a 1 ml volume of bioassay media and dialyzed overnight against a 1 l volume of DMEM media without supplements to remove the protein excipients. The media, containing either the fresh or lyophilized material, was added to ex-vivo cultures of rat (Sprague-Dawley) female, embryonic, urogenital ridges (E14.5) and incubated for 72 h at 37°C, 5% (v/v) CO2 in CMRL 1066 medium (Thermo Fisher Scientific) supplemented with 10% (v/v) female fetal bovine serum, 1% penicillin/streptomycin, 1% L-glutamine, 1% Fungizone (Thermo Fisher Scientific), and 5 nM testosterone. Ridges were fixed, sectioned and stained with haematoxylin and eosin and scored by two independent experienced operators. The study was performed in accordance with the Institutional Animal Care and Use Committee-approved experimental protocol 2014N000275 of the Massachusetts General Hospital.
Multi-method evaluation of the immunoreactivity of AMH in SS-581
To evaluate the content of AMH in terms of kit calibrators for existing immunoassays and immunoassays in development, six laboratories were provided with ampoules of the trial formulation, SS-581. The participants in the study were asked to perform the methods currently in use in their laboratory and to perform two independent runs of their method and, if capacity allowed, to analyse two ampoules of SS-581 in each run. In order to assess the parallelism of the response with the kit standards, participants were asked to measure a minimum of five concentrations, which were expected to give a response in the linear part of the dose-response curve. Participants were asked to provide details of the assay method(s) used, the diluent and dilution steps, together with all raw assay data, for central computation at NIBSC.
In addition to the ampouled preparations, participants were provided with a panel of six human serum samples coded S1 to S6 which, when measured in-house by manual AMH ELISA, contained AMH concentrations from 0.1 to 13.0 ng/ml. Serum samples were obtained from First Link UK (Wolverhampton, UK) and were certified non-reactive for HIV 1/2, HIV p24, HBsAg, anti-HCV and Syphilis TP by the supplier. The batches of frozen serum were aliquoted and stored at –80°C and distributed to participants on dry ice.
Data analysis
The AMH content of SS-581 was assumed to be 500 ng/ml. The raw data for each method data set were visually represented using GraphPad Prism 5 (Version 5.02, GraphPad Software Inc., La Jolla, USA). AMH content in terms of kit standard was determined by fitting a parallel-line model comparing log assay response to log concentration using CombiStats (Version 5.0, EDQM/Council of Europe). A geometric mean estimate of AMH content was determined for each method using Microsoft Excel 2010. Content estimates were included if four or more data points exhibited parallelism with the kit standard curve. Results from all valid assay runs were combined to generate unweighted geometric means for each laboratory and these laboratory means were used to calculate overall unweighted geometric mean immunoreactivity estimates of all methods and commercially available methods. Variability between laboratories was expressed using geometric coefficients of variation (GCV = {10s – 1} × 100%, where s is the standard deviation of the log10 transformed estimates).
An average reported value for serum samples S1 to S6 was determined from the assay data for each method. Summary results and the serum sample concentrations obtained by each method were visualized using GraphPad Prism 5. A geometric mean estimate of the AMH content of each serum sample was determined using Microsoft Excel 2010 and the geometric coefficient of variation (GCV%) calculated as described above.
Results
Characterization of the freeze-dried product
The material lyophilized to form robust loose, intact cakes. A total of 96 ampoules coded SS-581 were produced with a mean fill weight of 0.499 g (CV% 0.03%; n = 3), mean percentage oxygen of 0.61% (CV% 29.17%; n = 3) and mean residual moisture (% w/w) of 0.36% (n = 2). Using a commercially available manual ELISA, the AMH content of SS-581 was shown to be within 3% of that of the SS-581 formulation snap-frozen before lyophilization and stored at –30°C. The nominal content was estimated as 500 ng/amp.
Bioactivity of AMH in SS-581
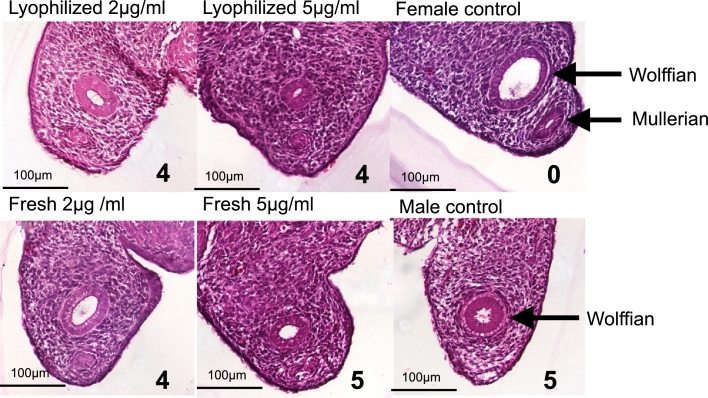
The lyophilized AMH in trial SS-581, after reconstitution and dialysis to remove the excipient components, was shown in the Müllerian duct regression bioassay to be bioactive with a potency similar to freshly purified AMH. When applied to ex-vivo cultures of fetal embryonic rat urogenital ridges (E14.5) for 72 h, AMH concentrations of 5 μg/ml caused near complete regression of the Müllerian duct (scores of 4–5, on a 0–5 scale with 5 representing complete regression), and retained this activity in further titration to 2 μg/ml, consistent with the bioactivity of purified, unlyophilized recombinant AMH. Representative sections (of n = 2) are shown in Figure 1.
Figure 1.
Bioactivity of freshly prepared and lyophilized recombinant AMH in the rat urogenital ridge bioassay. Representative transverse sections of rat embryonic urogenital ridge following incubation, ex vivo, with 2 μg/ml (left column) or 5 μg/ml (middle column) of lyophilized and reconstituted AMH (top row) or freshly purified AMH (bottom row). The regression scores presented in the bottom right quadrant of each image indicate the degree of biological activity from 0 to 5, where 0 represents no regression as observed in the untreated female ridge negative control (top right) and 5 indicates complete regression of the Müllerian duct as seen in the male untreated positive control (bottom right). Micrographs were taken at 200 × magnification; Müllerian and Wolffian ducts are indicated in control ridges with arrows.
Multi-method evaluation of the AMH content of SS-581 and serum samples S1–S6
Five of the six laboratories returned data from seven methods coded A–G (Table 1). These comprised two manual ELISA methods and five automated methods. Of the automated methods, two methods were the same assay on different platforms. Two automated methods were not commercially available at the time of the study. Two laboratories performed the same method (Method D); the data from these laboratories is presented as Di, provided by Laboratory 1 and Dii and Diii, provided by Laboratory 6. Dii and Diii vary in the nature of the sample diluent used (Table 1).
Table 1.
Methods contributing to assessment of the AMH content of the trial formulation and the diluents used in each method
| Method | Diluent | Runs | Amp/run |
|---|---|---|---|
| A | Sample diluent containing protein and preservative | 1 | 2 |
| B | Sample diluent containing BSA | 2 | 2 |
| C | Sample diluent containing BSA | 2 | 2 |
| Di (Lab 1) | Sample diluent containing BSA and preservative | 2 | 2 |
| Dii (Lab 6) | Sample diluent containing BSA and preservative | 1a | 1 |
| Diii (Lab 6) | PBS buffer containing 0.1% (w/v) BSA | 2a | 2 and 1 |
| E | Sample diluent containing protein, chemical stabilizers and preservative | 2 | 2 |
| F | Post-menopausal female serum | 2 | 2 |
| G | Buffer containing BSA | 2 | 2 |
BSA = bovine serum albumin; PBS = phosphate-buffered saline.
One run was used to compare SS-581 reconstituted and diluted in sample diluent with SS-581 reconstituted and diluted in PBS containing 0.1% BSA.
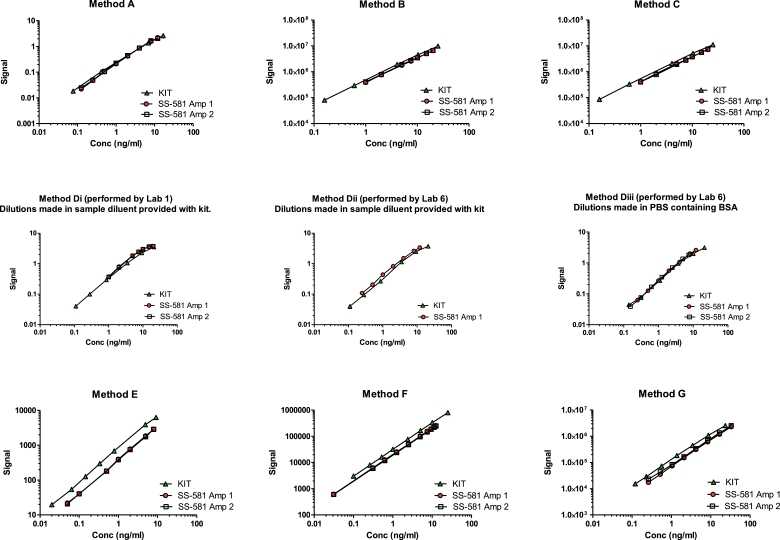
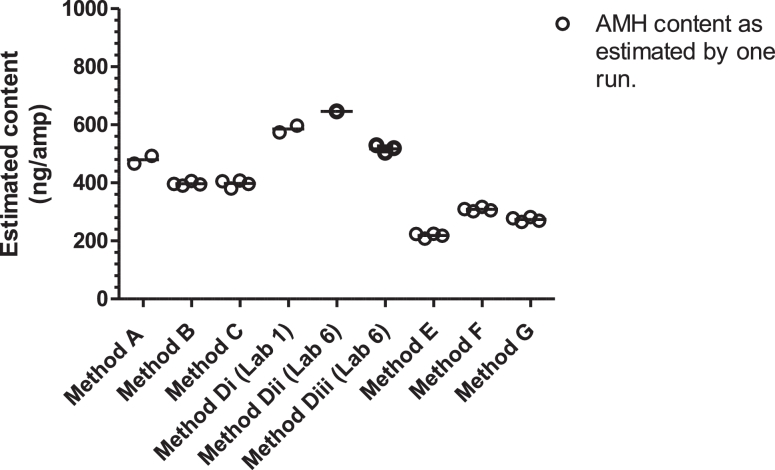
The recombinant human AMH in ampoules of SS-581 was recognized by all methods and in the majority of runs, the dose-response was parallel to method calibrators (Figure 2), allowing the determination of AMH content in terms of method calibrators as shown in Table 2 and Figure 3. The parallelism of the dose-response was borderline for Method Di, Run 1 (rows 1 and 2 of Table 2, column D1) and non-parallel for Method Di, Run 2 (rows 3 and 4 of Table 2, column Di), which may reflect the measurement of sample concentrations at the upper end of the kit standard curve. Although the identity of the methods has been anonymized for commercial reasons, Table 3 presents the geometric mean and variance of groups of methods, including those that were commercially available at the time of the study, those that are automated and those that are based on mAbs, F2B 12/H and F2B 7/A. The GCV of the mean content for these groups ranged from 26.67% to 39.95%.
Figure 2.
Representative data from Run 1 of Methods A to G as used to estimate the AMH content of trial preparation SS-581, in terms of the method calibrators.
Table 2.
Estimates of the AMH content of trial formulation SS-581, determined by Methods A to G and calculated in terms of kit calibrators
| AMH content per ampoule (ng/amp) | ||||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | Di | Dii | Diii | E | F | G |
| 466.30 | 390.70 | 396.66 | 597.31a | 646.41 | 519.18 | 224.00 | 317.37 | 265.05 |
| 493.08 | 405.99 | 380.67 | 573.99a | nd | 529.25 | 218.10 | 302.01 | 269.48 |
| nd | 394.36 | 405.82 | npb | nd | 503.53 | 224.42 | 309.87 | 282.45 |
| nd | 396.27 | 408.28 | npb | nd | nd | 208.08 | 305.03 | 278.16 |
| Geometric mean AMH content per ampoule (ng/amp) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 479.51 | 396.79 | 397.71 | 585.53 | 646.41 | 517.21 | 218.55 | 308.51 | 273.70 |
| Geometric mean of Method D | 580.64 | |||||||
AMH = anti-Müllerian hormone; nd = not determined; np = non-parallel.
Only four dilutions of SS-581 were in the linear part of the dose-response curve.
Dose-response was non-parallel to method calibrators.
Figure 3.
The estimated AMH content in trial preparation SS-581, in terms of kit standards as determined by Methods A to G.
Table 3.
Geometric mean and variance of (i) all methods, (ii) commercially available methods, (iii) automated methods and (iv) methods based on mAbs F2B 12/H and F2B 7/A
| Methodsa | Geometric mean | GCV% | n |
|---|---|---|---|
| All methods | 361.76 | 39.95 | 7 |
| Methods commercially available at the time of the study | 423.08 | 26.67 | 5 |
| Automated methods | 311.06 | 29.08 | 5 |
| Assays known to use mAbs, F2B 12/H and F2B 7/A | 410.90 | 29.79 | 4 |
The geometric mean of Method D was used to determine the mean and variance for method combinations where required.
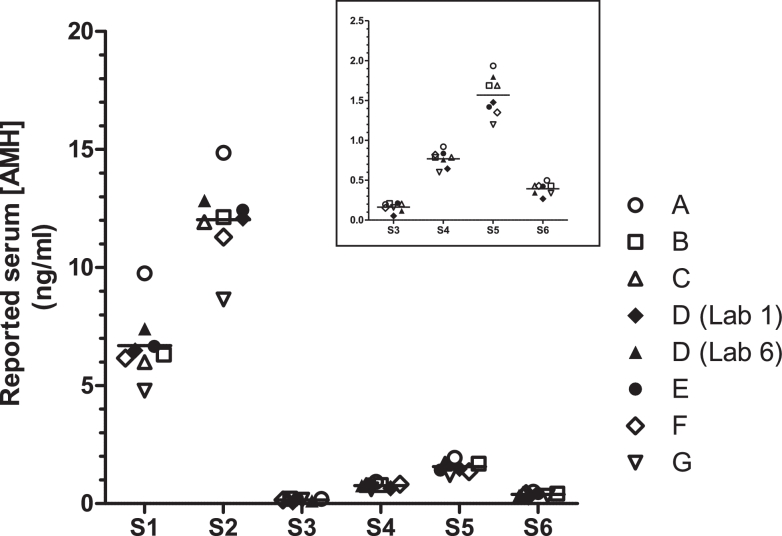
Participants were also asked to measure the AMH content in frozen aliquots of human serum, coded S1 to S6 (FIGURE 4). With the exception of serum sample S3, the measured AMH concentrations varied, with a GCV ranging from 14.90% to 22.35% (Table 4). The all-method mean for serum S3 demonstrated the highest variability, with a GCV% of 60.0%. This is likely to be due to the low concentration of AMH, which was below the lowest calibrator concentration for most methods.
Figure 4.
Mean estimates of the AMH content of serum samples S1 to S6 as reported by Methods A to G. The inset graph shows an expanded view of the data reported for serum samples S3–S6.
Table 4.
Reported estimates of the AMH concentration in serum samples S1–S6
| AMH (ng/ml) | ||||||
|---|---|---|---|---|---|---|
| Method | S1 | S2 | S3 | S4 | S5 | S6 |
| A | 9.75 | 14.86 | 0.20 | 0.92 | 1.94 | 0.50 |
| B | 6.31 | 12.13 | 0.21 | 0.79 | 1.69 | 0.43 |
| C | 5.99 | 11.92 | 0.20 | 0.79 | 1.69 | 0.43 |
| D (Lab 1) | 6.48 | 12.08 | 0.05 | 0.65 | 1.48 | 0.27 |
| D (Lab 6) | 7.40 | 12.84 | 0.11 | 0.76 | 1.79 | 0.34 |
| E | 6.65 | 12.42 | 0.21 | 0.84 | 1.42 | 0.42 |
| F | 6.16 | 11.29 | 0.15 | 0.82 | 1.35 | 0.43 |
| G | 4.79 | 8.65 | 0.16 | 0.60 | 1.20 | 0.34 |
| Geometric mean | 6.57 | 11.91 | 0.15 | 0.76 | 1.55 | 0.39 |
| GCV% | 22.35 | 16.44 | 60.01 | 14.90 | 17.42 | 21.65 |
Of the two methods (A and D) that provided the highest estimates for the AMH content of SS-581, Method A also demonstrated a similar trend in the reported concentration of AMH in the serum samples. Similarly, the AMH content of SS-581 estimated by Method G was less than the all-laboratory mean and the reported serum sample AMH concentrations were also below the median values. As such, it is probable that a reference material prepared from recombinant AMH would be commutable with these methods. Conversely, Methods D and E reported serum sample concentrations that were aligned with those reported by the majority of the method means but provided content estimates for SS-581 that were higher or lower, respectively, than the all-laboratory mean. It is clear that a comprehensive commutability study will be required during any collaborative assessment of a proposed international standard for AMH.
Discussion
Of the three methods currently used to assess ovarian reserve (follicular phase FSH, AMH and antral follicle count), AMH is thought to best represent the primordial follicle pool and thus provides the earliest and most sensitive indication of any diminished status of that reserve, as discussed by Tal and Seifer (2016). However, the authors emphasize that ‘assay variability and lack of a standardized international assay’ are the main limitations of this test.
This paper describes the evaluation of a proposed formulation of recombinant human AMH, coded SS-581, which will inform the approach to the future preparation of an international standard for AMH. It is clear from Figure 2 that AMH immunoreactivity is maintained upon lyophilization and in all methods tested, a linear dose-response is observed that is parallel to the method calibrators (Figure 2). Furthermore, the lyophilized material exhibits AMH bioactivity in the ex-vivo organ culture bioassay measuring regression of the Müllerian duct. This bioassay is considered the gold standard for testing the potency and specificity of AMH (Donahoe et al., 1977, Price et al., 1979) and the observed bioactivity suggests that conformational epitopes are maintained after lyophilization and reconstitution (Figure 1). This supports the proposed use of recombinant human AMH to develop a reference material to calibrate immunoassays of AMH.
The AMH content of trial preparation SS-581 was measured by two manual methods, two automated methods known to be commercially available (one of which was assessed on two platforms), and two automated assays that were in development at the time of the study. As can be seen from Table 3, the AMH content of SS-581 varied with a GCV% of 39.95% across all assay methods, although it must be noted that this decreased to 29.08% for automated immunoassay platforms and to 26.67% for the commercially available methods. The assays that are known to use monoclonal antibodies F2B 12/H and F2B 7/A also demonstrated variability, with a GCV of 29.79%, suggesting that epitope recognition may not be the sole contributor to the observed variation and that assay calibration, format and the kinetics of antibody binding may also influence the reported AMH concentrations. The potential effect of the choice of diluent must also be considered. Pearson et al. (2016) diluted serum samples in either pooled patient serum, with a low AMH concentration, or in the provided sample diluent, and compared the resultant AMH concentrations with those of the undiluted samples. They reported that the AMH concentrations of the dilutions made in pooled serum showed less deviation from the value obtained for the neat samples compared with the dilutions made in the kit diluent (–2.56 to 5.21% versus –3.63 to 13.31%).
The introduction of an international standard aims to improve the comparability of results between laboratories, providing confidence in reported results and reference ranges. This is usually achieved by the designation of an international unit (IU) of immunoreactivity, a consensus value derived from evaluation by multiple laboratories in a collaborative study. Adoption of the international standard then serves to harmonize current and new methods through recalibration to the agreed value. Thus, an approach to the standardization of AMH assays would be to introduce an IU of AMH immunoreactivity, based on a preparation similar to our trial preparation, SS-581. However, AMH presents a challenge to this approach as, although it is a large, complex glycoprotein, current methods report in mass units. Introduction of another unit of AMH immunoreactivity is likely to cause confusion and will encourage the use of method-specific conversion factors to report in the expected units and to account for differences in the performance of assay methods. These can result from differences in antibody binding, calibrators, matrix effects and the use of protocols in which antibody binding does not reach equilibrium.
Definition of a standard in mass units requires traceability to the SI unit of mass by the use of a physicochemical reference method. As described below, this precludes the use of methods that rely on the binding of an antibody. The guidance for the preparation of a reference material on behalf of WHO with a value assigned in molar or mass units states that ‘the principles outlined in ISO 17511 should be followed. This will necessitate the existence and use of an appropriate single reference method and an assignment of uncertainty, derived from calibration data. Such a reference method should not be a biological assay since the factors that affect the results of such assays cannot be fully described. Where they are used, SI units assigned to biological reference standards should be derived from and traceable to a physico-chemical procedure’ (WHO, 2006). Examples of such procedures for biological materials include weighing, UV absorbance and amino acid analysis. Mass units have been successfully assigned to international standards for small, well-defined biological analytes such as peptides, providing that they can be formulated and lyophilized using small-molecule excipients. In these cases, calibration has been achieved using a two-stage process of (i) assigning a mass value to a primary calibrant by amino acid analysis using NIST-traceable reference standards and (ii) determination of the content of the international standard by HPLC in terms of the primary calibrant. It must also be noted that this approach thereby assumes that all the detected mass is active in the eventual biological assay system.
It is clear that this approach will be challenging for AMH, which is a relatively large, dimeric protein with an undefined proportion of its mass contributed by glycosylation and which exists as a mixture of cleaved and uncleaved forms. Furthermore, the necessity for a protein-based excipient to ensure the stability of AMH during filling, lyophilization and reconstitution precludes the use of HPLC methods for the calibration of the candidate standard.
The use of tryptic digestion and isotope dilution mass spectrometry is currently being investigated at NIBSC to quantify AMH polypeptide in formulated lyophilized preparations by determination of the molar quantities of selected AMH tryptic peptides. However, recognizing that various calibration exercises have occurred during the development of AMH immunoassay methods and given that there has been no single, universally accepted reference material, there is the possibility that a ‘true’ value assigned by a physicochemical reference method will not agree with all or any of the current methods. In such a case, the approach that provides the best outcome for patients must be identified and proposed.
This study was not designed to assess the commutability of SS-581. Such an assessment would require considerably more serum samples across the full range of AMH measurements and the measurement in triplicate of defined dilutions of the reference material by each method. The inclusion of a small number of serum samples in the study suggested that a recombinant reference material may be commutable with most of the methods tested. However, it is possible that differences in antibody recognition between native and recombinant AMH, as well as differences in calibration and assay protocol (such as length of antigen–antibody incubations) may affect the commutability of a reference material based on recombinant AMH. Groome (2015) commented that the rate of association and disassociation of antibodies cannot be assumed to be the same between non-human and human AMH. The assessment of commutability in a future collaborative study is further complicated if assays respond differently to AMH provided as frozen aliquots compared with fresh serum, as suggested by Li et al. (2016).
An alternative source of AMH for a future international standard is native, human AMH in serum. Intuitively, this may appear more suitable to harmonize assays but it is not without challenges as an approach. A minimum volume of 2 l of serum will be required, ideally with an AMH content that is at least four-fold higher than the highest standard used in commercially available methods. Because such levels are only routinely found in boys under 12 years of age or in male cord blood (Aksglaede et al., 2010), there would be challenges in acquiring the required volume. More importantly, there is no guarantee that a lyophilized, serum-based standard will be more commutable than a standard prepared with recombinant AMH. Assignment of AMH content to a serum-based standard would rely solely on a consensus value derived from the immunoassays in use at the time of calibration. Thus, any proposal to introduce a serum-based reference material requires consideration of the eventual replacement of the standard in 5–10 years, because any subsequent serum pool may behave very differently in the contemporary assays compared with the initial serum standard.
The clinical use of AMH immunoassays is maturing. As AMH is a relatively non-fluctuating, sensitive serum marker, measurements of AMH will increasingly contribute to the diagnosis and treatment decisions for women with diminished ovarian reserve. Although no single measure of ovarian reserve is used in isolation, internationally standardized assays would provide confidence in cited reference ranges and cut-off values. Currently, these are method-dependent and may have been derived by non-automated methods (Tal and Seifer, 2017). An international standard will support the development of AMH immunoassays that are calibrated to recombinant human AMH and the data presented here suggest that a lyophilized preparation of recombinant human AMH, similar to that trialled here, may be suitable. To this end, NIBSC has prepared a batch of 3600 ampoules, coded 16/190, which will be evaluated through an international collaborative study. The study will include an assessment of commutability through the concomitant measurement of the AMH content of dilutions of the candidate reference material and of aliquots of serum and plasma samples provided to each participant. Agreement between methods is reportedly improving and confidence in reported AMH values has increased. In addition, a specific AMH assay is being evaluated as a companion diagnostic to inform the dosing of a recombinant FSH product (Nyboe Andersen et al., 2017). Therefore, it is apparent that the eventual introduction of an international standard for recombinant human AMH will require consideration of these developments.
Declaration
The authors report no financial or commercial conflicts of interest.
Biography

Jackie Ferguson graduated from the University of Bath in 1995 with a PhD in Biochemistry through research into protein thermostability. With subsequent experience in industry, she was appointed to NIBSC in 2011 and is involved in multiple projects to develop international standards on behalf of the World Health Organization.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.rbmo.2018.08.012.
Appendix. Supplementary materials
References
- Aksglaede L., Sorensen K., Boas M., Mouritsen A., Hagen C.P., Jensen R.B., Petersen J.H., Linneberg A., Andersson A.M., Main K.M., Skakkebæk N.E., Juul A. Changes in anti-Mullerian hormone (AMH) throughout the life span: a population-based study of 1027 healthy males from birth (cord blood) to the age of 69 years. J Clin Endocrinol Metab. 2010;95:5357–5364. doi: 10.1210/jc.2010-1207. [DOI] [PubMed] [Google Scholar]
- Anckaert E., Öktem M., Thies A., Cohen-Bacrie M., Daan N.M., Schiettecatte J., Müller C., Topcu D., Gröning A., Ternaux F., Engel C., Engelmann S., Milczynski C. Multicentre analytical performance evaluation of a fully automated anti-Mullerian hormone assay and reference interval determination. Clin Biochem. 2016;49:260–267. doi: 10.1016/j.clinbiochem.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Bersinger N.A., Wunder D., Birkhauser M.H., Guibourdenche J. Measurement of anti-mullerian hormone by Beckman Coulter ELISA and DSL ELISA in assisted reproduction: differences between serum and follicular fluid. Clin Chim Acta. 2007;384:174–175. doi: 10.1016/j.cca.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Budzik G.P., Powell S.M., Kamagata S., Donahoe P.K. Mullerian inhibiting substance fractionation by dye affinity chromatography. Cell. 1983;34:307–314. doi: 10.1016/0092-8674(83)90161-7. [DOI] [PubMed] [Google Scholar]
- Cate R.L., Mattaliano R.J., Hession C., Tizard R., Farber N.M., Cheung A., Ninfa E.G., Frey A.Z., Gash D.J., Chow E.P., Fisher R.A., Bertonis J.M., Torres G., Wallner B.P., Ramachandran K.L., Ragin R.C., Manganaro D.T., Donahoe P.K. Isolation of the bovine and human genes for Mullerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45:685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- Demirdjian G., Bord S., Lejeune C., Masica R., Riviere D., Nicouleau L., Denizot P., Marquet P.Y. Performance characteristics of the Access AMH assay for the quantitative determination of anti-Mullerian hormone (AMH) levels on the Access* family of automated immunoassay systems. Clin Biochem. 2016;49:1267–1273. doi: 10.1016/j.clinbiochem.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Dewailly D., Andersen C.Y., Balen A., Broekmans F., Dilaver N., Fanchin R., Griesinger G., Kelsey T.W., La Marca A., Lambalk C., Mason H., Nelson S.M., Visser J.A., Wallace W.H., Anderson R.A. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20:370–385. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- Donahoe P.K., Ito Y, Hendren W.H. A graded organ culture assay for the detection of Mullerian inhibiting substance. J Surg Res. 1977;23:141–148. doi: 10.1016/0022-4804(77)90202-5. [DOI] [PubMed] [Google Scholar]
- Freour T., Mirallie S., Bach-Ngohou K., Denis M., Barriere P., Masson D. Measurement of serum anti-Mullerian hormone by Beckman Coulter ELISA and DSL ELISA: comparison and relevance in assisted reproduction technology (ART) Clin Chim Acta. 2007;375:162–164. doi: 10.1016/j.cca.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Gassner D., Jung R. First fully automated immunoassay for anti-Mullerian hormone. Clin Chem Lab Med. 2014;52:1143–1152. doi: 10.1515/cclm-2014-0022. [DOI] [PubMed] [Google Scholar]
- Gopal V.S., Ajay K. Antibody compositions and immunoassay methods to detect isoforms of anti-mullerian hormone. WIPO PCT Patent application. 2014 No. WO2014074835 A2. [Google Scholar]
- Groome NP. The design features and performance of a state-of-the-art fully-automated anti-Mullerian hormone immunoassay for the Beckman Access family of immunoassay systems. In: Coulter B., editor. 2015. http://wwwbeckmancoulter-amhcom/en/wp-content/uploads/Access-AMH-Technical-Bulletin-14–07–2015pdf (Beckman Coulter) [Google Scholar]
- Groome N.P., Cranfield M., Themmen A.P.N., Savjani G.V., Mehta K. Immunological assay and antibodies for anti-Mullerian hormone. US Patent. 2011 No. US7897350. [Google Scholar]
- Josso N., Cate R.L., Picard J.Y., Vigier B., di Clemente N., Wilson C., Imbeaud S., Pepinsky R.B., Guerrier D., Boussin L., Legeai L., Carre-Eusebe D. Anti-mullerian hormone: the Jost factor. Recent Prog Horm Res. 1993;48:1–59. doi: 10.1016/b978-0-12-571148-7.50005-1. [DOI] [PubMed] [Google Scholar]
- Kumar A., Kalra B., Patel A., McDavid L., Roudebush WE. Development of a second generation anti-Mullerian hormone (AMH) ELISA. J Immunol Methods. 2010;362:51–59. doi: 10.1016/j.jim.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Li H.W., Wong B.P., Ip W.K., Yeung W.S., Ho P.C., Ng E.H. Comparative evaluation of three new commercial immunoassays for anti-Mullerian hormone measurement. Hum Reprod. 2016;31:2796–2802. doi: 10.1093/humrep/dew248. [DOI] [PubMed] [Google Scholar]
- Long W.Q., Ranchin V., Pautier P., Belville C., Denizot P., Cailla H., Lhomme C., Picard J.Y., Bidart J.M., Rey R. Detection of minimal levels of serum anti-Mullerian hormone during follow-up of patients with ovarian granulosa cell tumour by means of a highly sensitive enzyme-linked immunosorbent assay. J Clin Endocrinol Metab. 2000;85:540–544. doi: 10.1210/jcem.85.2.6378. [DOI] [PubMed] [Google Scholar]
- MHRA Field Safety Notice, “AMH Gen II ELISA kit (part no A79765)” MHRA 2013 Ref:2013/007/004/081/029. http://webarchive.nationalarchives.gov.uk/20131203023256/http://www.mhra.gov.uk/PrintPreview/DefaultSP/CON297493
- Monniaux D., Clement F., Dalbies-Tran R., Estienne A., Fabre S., Mansanet C., Monget P. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: what is the link. Biol Reprod. 2014;90:85. doi: 10.1095/biolreprod.113.117077. [DOI] [PubMed] [Google Scholar]
- Nelson S.M., Pastuszek E., Kloss G., Malinowska I., Liss J., Lukaszuk A., Plociennik L., Lukaszuk K. Two new automated, compared with two enzyme-linked immunosorbent, antimullerian hormone assays. Fertil Steril. 2015 doi: 10.1016/j.fertnstert.2015.06.024. 104:1016–1021 e1016. [DOI] [PubMed] [Google Scholar]
- Nyboe Andersen A., Nelson S.M., Fauser B.C., Garcia-Velasco J.A., Klein B.M., Arce J.C., the ESTHER-1 study group Individualized versus conventional ovarian stimulation forin vitro fertilization: a multicentre, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil Steril. 2017;107:387–396. doi: 10.1016/j.fertnstert.2016.10.033. [DOI] [PubMed] [Google Scholar]
- Pankhurst M.W., Chong Y.H., McLennan I.S. Relative levels of the proprotein and cleavage-activated form of circulating human anti-Mullerian hormone are sexually dimorphic and variable during the life cycle. Physiol Rep. 2016;4:e12783. doi: 10.14814/phy2.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankhurst M.W., McLennan I.S. Human blood contains both the uncleaved precursor of anti-Mullerian hormone and a complex of the NH2- and COOH-terminal peptides. Am J Physiol Endocrinol Metab. 2013;305:E1241–E1247. doi: 10.1152/ajpendo.00395.2013. [DOI] [PubMed] [Google Scholar]
- Pearson K., Long M., Prasad J., Wu Y.Y., Bonifacio M. Assessment of the Access AMH assay as an automated, high-performance replacement for the AMH Generation II manual ELISA. Reprod Biol Endocrinol. 2016;14:8. doi: 10.1186/s12958-016-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin D., Hoang M., Nicolaou F., Hendren K., Benedict L.A., Al-Moujahed A., Sosulski A., Marmalidou A., Vavvas D., Donahoe P.K. An albumin leader sequence coupled with a cleavage site modification enhances the yield of recombinant C-terminal Mullerian Inhibiting Substance. Technology. 2013;1:63–71. doi: 10.1142/S2339547813500076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre A., Racine C., Rey R., Fanchin R., Taieb J., Cohen-Tannoudji J., Carmillo P., Pepinsky R.B., Cate R.L., di Clemente N. Most cleaved anti-Mullerian hormone binds its receptor in human follicular fluid but little is competent in serum. J Clin Endocrinol Metab. 2016;101:4618–4627. doi: 10.1210/jc.2016-1742. [DOI] [PubMed] [Google Scholar]
- Price J.M., Donahoe P.K., Ito Y. Involution of the female Mullerian duct of the fetal rat in the organ-culture assay for the detection of Mullerian Inhibiting Substance. Am J Anat. 1979;156:265–284. doi: 10.1002/aja.1001560207. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Hayashi M., Manganaro T.F., Donahoe P.K. The ontogeny of mullerian inhibiting substance in granulosa cells of the bovine ovarian follicle. Biol Reprod. 1986;35:447–453. doi: 10.1095/biolreprod35.2.447. [DOI] [PubMed] [Google Scholar]
- Tal R., Seifer D.B. Ovarian reserve testing: a user's guide. Am J Obstet Gynecol. 2017;217:129–140. doi: 10.1016/j.ajog.2017.02.027. [DOI] [PubMed] [Google Scholar]
- Van Helden J., Weiskirchen R. Performance of the two new fully automated anti-Mullerian hormone immunoassays compared with the clinical standard assay. Hum Reprod. 2015;30:1918–1926. doi: 10.1093/humrep/dev127. [DOI] [PubMed] [Google Scholar]
- Vigier B., Picard J.Y., Tran D., Legeai L., Josso N. Production of anti-Mullerian hormone: another homology between Sertoli and granulosa cells. Endocrinology. 1984;114:1315–1320. doi: 10.1210/endo-114-4-1315. [DOI] [PubMed] [Google Scholar]
- Visser, JAL, JSE, McLuskey, A, Louwers, YV, Van Dorp, W, Themmen APN, Kalra, B, Patel AS, Kumar, A. Development of a sensitive and human specific AMH Chemiluminescence assay for the quantitative measurement of AMH in serum, plasma and follicular fluid. ENDO 2013 (San Francisco, USA).
- WHO Expert Committee on Standardization; fifty-fifth report. World Health Organization 2006; 932: Annex 2, 75–130. Geneva, Switzerland.
- WHO Expert Committee on Standardization; sixty-fifth report. World Health Organization 2015; 993:48. Geneva, Switzerland. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.