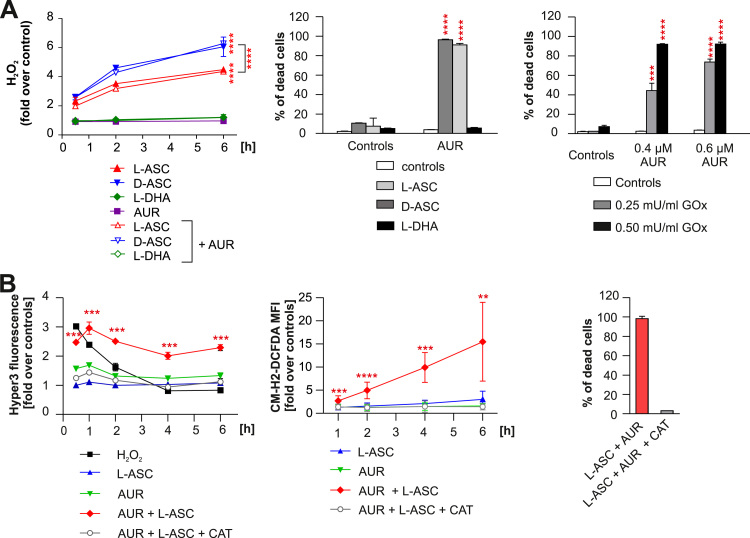
Fig. 3.
L-ASC and AUR combination triggers the intracellular accumulation of H2O2 and H2O2-dependent cell death. A. Cell-free culture medium supplemented with 200 µM L-ASC, 200 µM D-ascorbate (D-ASC) or 200 µM L-dehydroascorbate (L-DHA) alone or in combination with 0.5 µM AUR was incubated for indicated time-points. PY1 probe was added to the medium at 10 μM final concentration. The fluorescence was measured using the excitation wavelength 514 nm and emission wavelength 550 nm. The amount of generated H2O2 was normalized to control (DMSO). The representative result (out of two independent experiments) is shown as means ± SD (n = 4). Statistical significance was evaluated using 2-way ANOVA with Tukey's multiple comparisons test and is shown for control vs L-ASC, control vs D-ASC and L-ASC vs D-ASC groups only; **** p < 0.0001 (left panel). Raji cells were incubated with 200 µM L-ASC, 200 µM D-ASC or 200 µM L-DHA alone or in combination with 0.4 µM AUR for 48 h. Thereafter the cells were stained with PI and their viability was assessed by flow cytometry. The results are shown as a mean percentage of dead cells + SD from two independent experiments. Statistical significance was evaluated using 1-way ANOVA test with Tukey's correction in L-ASC, D-ASC and L-DHA only groups and the corresponding combinations, ****p < 0.0001 (middle panel). Raji cells were incubated with glucose oxidase (GOx) and AUR at indicated concentrations, alone or in combination. After 48 h, the cells were stained with PI and the percentage of dead cells was evaluated by flow cytometry. Results are shown as means of three independent experiments + SD. Statistical significance was evaluated using 1-way ANOVA test with Tukey's correction in GOx only groups and the corresponding combinations with AUR, ***p < 0.001, ****p < 0.0001 (right panel). B. Raji cells expressing HyPer3 were incubated with 200 µM L-ASC, 0.5 µM AUR, or the combination of both. Where indicated, catalase (CAT,100 µg/ml) was added 30 min prior to addition of L-ASC and AUR. The intensity of green fluorescence was assessed at indicated time points by flow cytometry. The results are presented as fold change over untreated controls, as means from two experiments ± SD, n = 4. Statistical significance in control vs L-ASC+AUR group only for each time point was assessed using 1-way ANOVA test with Dunnett's post-hoc test; ***p < 0.001 (left panel). Raji cells pre-stained with 1.5 µM CM-H2-DCFDA were incubated with 200 µM L-ASC, 0.5 µM AUR, or their combination. The intensity of green fluorescence of all living cells, gated based on SSC and FSC parameters, was assessed by flow cytometry at indicated time points. CAT (100 µg/ml) was added 30 min prior to addition of L-ASC and AUR to the indicated groups. The results are presented as fold over untreated control. Means of three independent experiments ± SD are presented. Statistical significance between each experimental group and the DMSO-treated control for each time-point was assessed using 1-way ANOVA with Dunnett's post-hoc test; **p < 0.01, ***p < 0.001, ****p < 0.0001 (middle panel). The viability of Raji cells incubated 48 h with the combination of 200 µM L-ASC and 0.5 µM AUR ± 100 µg/ml CAT was assessed by flow cytometry after PI staining. Bars present means of two independent experiments + SD (right panel).