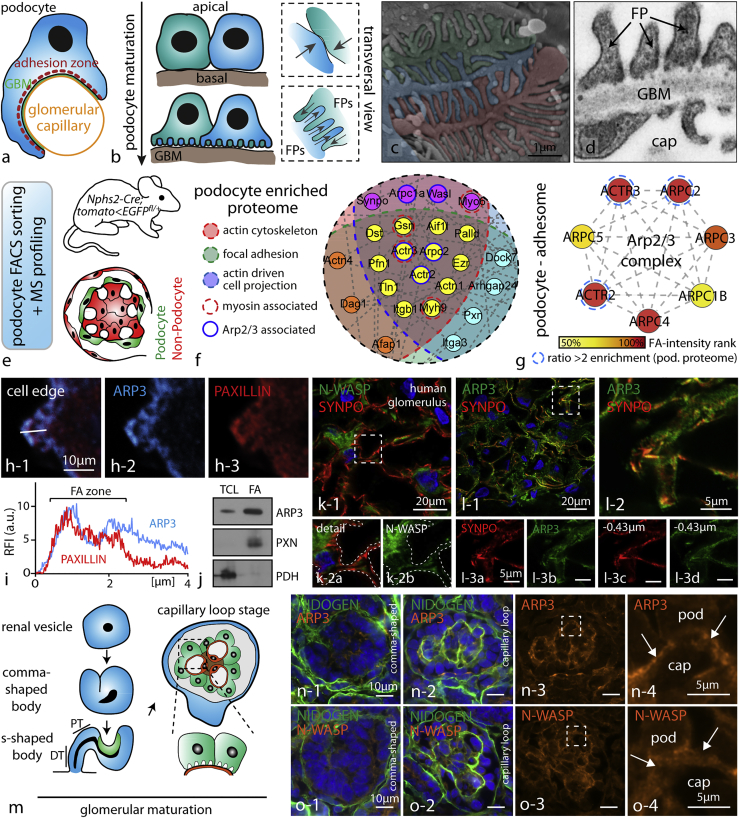
Figure 1.
The Arp2/3 Complex Presents a Central Node in the Network of Cytoskeletal Proteins in Podocytes
(A) Schematic depicting how podocytes reside on the outer surface of glomerular capillaries.
(B) During glomerular maturation podocytes undergo profound cell shape changes (GBM, glomerular basement membrane; FP, podocyte foot process).
(C) Colorized scanning electron microscopy (SEM) image of podocyte protrusions illustrating the complex interdigitating network of podocyte FPs covering glomerular capillaries.
(D) Transmission electron microscopy (TEM) demonstrates FPs attaching to the glomerular basement membrane (GBM; cap - glomerular capillary). Slits between individual FPs are bridged by specialized cell-cell contacts known as slit diaphragms.
(E) Schematic describing the strategy for isolation of genetically labeled primary podocytes and subsequent mass spectrometry (MS) analysis. Following single-cell isolation and further FACS-based purification, respective cell populations are analyzed.
(F) Proteome network analysis of podocyte-enriched proteins revealed high abundance of proteins contributing to distinct functional subsets such as actin binding or focal adhesion-associated proteins. Filtering of the enriched proteins for the indicated functional subsets confirmed components of the Arp2/3 complex as a central node (color coded: enrichment scores and functional groups are indicated in Table S1; proteins were defined as podocyte enriched by a detection ratio >2 for podocytes to non-podocyte glomerular cells).
(G) Focal adhesome proteomics generated out of immortalized human podocytes detected multiple members of the Arp2/3 complex as enriched components of the focal adhesome (FA) (enrichment scores are indicated in Table S2; proteins were hierarchically ranked according to their detection scores).
(H and I) Immunofluorescence of spreading primary wild-type podocytes revealed co-localization of ARP3 and the focal adhesion protein PAXILLIN during early spreading phases (white line indicates region for intensity line scan analysis; RFI, relative fluorescence intensity; FA, focal adhesion).
(J) Western blot analysis of focal adhesion (FA)-enriched cell fractions or total cell lysate (TCL) confirmed ARP3 as a FA-enriched protein in podocytes. PXN (Paxillin) and PDH (Pyruvate dehydrogenase E1) were used to demonstrate successful separation of the focal adhesion fraction from the whole cell compartment (see also Figure S2).
(K) Confocal laser scanning microscopy of human glomeruli confirmed distinct localization of the nucleation-promoting factor N-WASP in the podocyte compartment (dashed line boxes indicate areas of higher magnification inserts).
(L) A similar enrichment was also observed for ARP3 employing confocal laser scanning microscopy (inserts in (L) show different levels of z stack series 0.43 micrometers apart; dashed outlines indicate the podocyte compartment; synpatopodin (SYNPO) was used as a marker for the podocyte compartment).
(M) Schematic depicting stages of glomerular development (podocyte progenitors and podocytes are shown in green; endothelial cells and capillary loops are depicted in orange).
(N and O) At late capillary loop stage, ARP3 (N) and N-WASP (O) accumulated at the basal compartment of maturating podocytes, where podocyte FP formation takes place (in earlier developmental stages, this prominent accumulation was not apparent; dashed line boxes indicate inserts; arrows indicating the basal podocyte compartment; pod, podocyte; cap, capillary; NIDOGEN was employed as a marker of the glomerular basement membrane; mice glomeruli were analyzed at p0).